Abstract
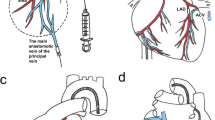
Transcoronary gene delivery represents a desirable option to achieve global myocardial transgene expression but still requires aggressive surgical preparation in rodents. We therefore developed a catheter-based approach for cardiac gene transfer in the closed chest rat. A double-lumen balloon catheter was used to create aortic occlusion for specific infusion of adenoviral vectors carrying a β-galactosidase transgene (1 × 1011 PFU) into the coronaries. Simultaneously, venous return was obstructed by a second balloon catheter in the right atrium. To prolong viral incubation time, we induced a transient cardiac arrest (2 and 5 min) by a combination of acetylcholine and the β-receptor antagonist, esmolol. At 72 h after transfection, the hearts showed a homogeneous and widespread β-galactosidase expression, and the transduction efficiency increased and up to about 43% of cardiac myocytes (histochemistry) with a 400-fold increase of β-galactosidase activity (luminescence assay) compared to sham-operated hearts. Pharmacological treatment aimed at increasing vascular permeability (SNAP and histamine) did not bring about synergistic effects on transfection efficiency. In addition, the method using high intracoronary pressure delivery (>300 mmHg) in a single-pass manner resulted in rather sparse β-galactosidase expression in the myocardium (3–5% of cardiac myocytes). Therefore, the percutaneous gene delivery system described here provides a simple and minimally invasive procedure that represents a novel strategy for a homogeneous and highly efficient in vivo gene transfer to rodent hearts. Our results also suggest that prolongation of viral incubation time is an effective means for achieving highly efficient myocardial gene transduction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Isner JM . Myocardial gene therapy. Nature 2002; 415: 234–239.
Crystal RG . Pumping up the heart. Gene Therapy 2003; 10: 2–3.
Ikeda Y et al. Restoration of deficient membrane proteins in the cardiomyopathic hamster by in vivo cardiac gene transfer. Circulation 2002; 105: 502–508.
Del Monte F et al. Improvement in survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase in a rat model of heart failure. Circulation 2001; 104: 1424–1429.
Maurice JP et al. Enhancement of cardiac function after adenoviral-mediated in vivo intracoronary beta2-adrenergic receptor gene delivery. J Clin Invest 1999; 104: 21–29.
Tevaearai HT et al. Myocardial gene transfer and overexpression of beta2-adrenergic receptors potentiates the functional recovery of unloaded failing hearts. Circulation 2002; 106: 124–129.
Hajjar RJ et al. Modulation of ventricular function through gene transfer in vivo. Proc Natl Acad Sci USA 1998; 95: 5251–5256.
Kypson A et al. Adenovirus-mediated gene transfer of the beta2-adrenergic receptor to donor hearts enhances cardiac function. Gene Therapy 1999; 6: 1298–1304.
Barr E et al. Efficient catheter-mediated gene transfer into the heart using replication-defective adenovirus. Gene Therapy 1994; 1: 51–58.
Shah AS et al. Intracoronary adenovirus-mediated delivery and overexpression of the beta(2)-adrenergic receptor in the heart: prospects for molecular ventricular assistance. Circulation 2000; 101: 408–414.
Beeri R et al. New efficient catheter-based system for myocardial gene delivery. Circulation 2002; 106: 1756–1759.
Donahue JK et al. Acceleration of widespread adenoviral gene transfer to intact rabbit hearts by coronary perfusion with low calcium and serotonin. Gene Therapy 1998; 5: 630–634.
Logeart D et al. How to optimize in vivo gene transfer to cardiac myocytes: mechanical or pharmacological procedures? Hum Gene Ther 2001; 12: 1601–1610.
Wright MJ, Wightman LM, Latchman DS, Marber MS . In vivo myocardial gene transfer: optimization and evaluation of intracoronary gene delivery in vivo. Gene Therapy 2001; 8: 1833–1839.
Boekstegers P et al. Myocardial gene transfer by selective pressure-regulated retroinfusion of coronary veins. Gene Therapy 2000; 7: 232–240.
Schmitz B et al. Recovery of the rodent brain after cardiac arrest: a functional MRI study. Magn Reson Med 1998; 39: 783–788.
Donahue JK et al. Ultrarapid, highly efficient viral gene transfer to the heart. Proc Natl Acad Sci USA 1997; 94: 4664–4668.
Skepper JN et al. Cytochemical demonstration of sites of hydrogen peroxide generation and increased vascular permeability in isolated pig hearts after ischaemia and reperfusion. Microsc Res Tech 1998; 42: 369–385.
Nagata K, Marban E, Lawrence JH, Donahue JK . Phosphodiesterase inhibitor-mediated potentiation of adenovirus delivery to myocardium. J Mol Cell Cardiol 2001; 33: 575–580.
Ede M et al. Beyond hyperkalemia: beta-blocker-induced cardiac arrest for normothermic cardiac operations. Ann Thorac Surg 1997; 63: 721–727.
Acknowledgements
This work was supported by grant 9772136 from the Forschungskommission of the Heinreich-Heine-Universität Düsseldorf. We wish to thank Mrs S Küsters for technical assistance and Dr G Kappert for lactate and creatinine measurements.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ding, Z., Fach, C., Sasse, A. et al. A minimally invasive approach for efficient gene delivery to rodent hearts. Gene Ther 11, 260–265 (2004). https://doi.org/10.1038/sj.gt.3302167
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302167
Keywords
This article is cited by
-
Cardiac gene therapy: are we there yet?
Gene Therapy (2016)
-
Ventricular Fibrillation-Induced Cardiac Arrest in the Rat as a Model of Global Cerebral Ischemia
Translational Stroke Research (2013)
-
Concomitant Intravenous Nitroglycerin With Intracoronary Delivery of AAV1.SERCA2a Enhances Gene Transfer in Porcine Hearts
Molecular Therapy (2012)
-
Augmentation of AAV-mediated cardiac gene transfer after systemic administration in adult rats
Gene Therapy (2008)
-
Gene Therapy Progress and Prospects: Electroporation and other physical methods
Gene Therapy (2004)