Abstract
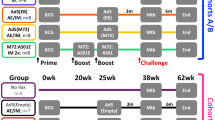
The prevention of Mycobacterium tuberculosis (M. tuberculosis) reactivation would greatly reduce the incidence of the disease, particularly among the elderly. Here, we evaluated the efficacy of DNA vaccine in combination with a conventional TB chemotherapy on the prevention of M. tuberculosis reactivation. Mice were treated with isoniazid and pyrazinamide for 3 months from 4 weeks after aerosol infection with M. tuberculosis H37Rv. During this period of chemotherapy, DNA immunization was performed three times monthly with an antigen 85A (Ag85A) DNA or an IL-12 mutant (IL-12N220L) DNA, which is known to lead to a reduction in the secretion of the p40 subunit, but not of a bioactive IL-12p70. The reactivation of M. tuberculosis was dramatically reduced in mice treated with either Ag85A DNA (P<0.01) or IL-12N220L DNA (P<0.05) in combination with chemotherapy, compared with control mice receiving only chemotherapy. Ag85A DNA vaccine showed higher IFN-γ responses to Ag85A protein, but a lower response to culture filtrate than IL-12N220L DNA vaccine. In addition, Ag85A DNA vaccine prevented the reactivation of M. tuberculosis more efficiently than IL-12N220L DNA vaccine, indicating that Ag85A-specific IFN-γ response might correlate with M. tuberculosis control. This study suggests that immunotherapy using Ag85A or IL-12N220L DNA vaccine combined with conventional chemotherapy might be effective clinically for the prevention of tuberculosis reactivation and may offer a more effective cure for humans than chemotherapy alone.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kochi A, Nunn P, Dye C, Tayler E . Global burden of disease. Lancet 1997; 350: 142 (letter).
Toossi Z, Ellner JJ . Pathogenesis of tuberculosis. In: Friedman LN (ed). Tuberculosis: Current Concepts and Treatment. CRC Press: New York, 2001, pp 19–47.
Rieder HL . Interventions for Tuberculosis Control and Elimination. International Union Against Tuberculosis and Lung Disease: Paris, 2002.
Broekmans JE . Control strategies and programme management. In: Porter JDH, McAdam KPWJ (eds). Tuberculosis: Back to the Future. John Wiley and Sons: New York, 1994, pp 171–192.
Chaulk CP, Kazandjian VA . Directly observed therapy for treatment completion of pulmonary tuberculosis: consensus statement of the public health tuberculosis guidelines panel. J Am Med Assoc 1998; 279: 943–948.
Mitchison DA . The action of antituberculosis drugs in short-course chemotherapy. Tubercle 1985; 66: 219–225.
Young DB, Robertson DB . Approaches to combat tuberculosis. Curr Opin Biotechnol 1998; 9: 650–652.
Andersen P . TB vaccines: progress and problems. Trends Immunol 2001; 22: 160–168.
Boom WH, Wallis RS, Chervenak KA . Human Mycobacterium tuberculosis-reactive CD4+ T-cell clones: heterogeneity in antigen recognition, cytokine production, and cytotoxicity for mononuclear phagocytes. Infect Immun 1991; 59: 2737–2743.
Kaufmann SH . Immunity to intracellular bacteria. Annu Rev Immunol 1993; 11: 129–163.
Tan JS et al. Human alveolar T lymphocyte responses to Mycobacterium tuberculosis antigens: role for CD4+ and CD8+ cytotoxic T cells and relative resistance of alveolar macrophages to lysis. J Immunol 1997; 159: 290–297.
Bonato VLD et al. Identification and characterization of protective T cells in hsp65 DNA-vaccinated and Mycobacterium tuberculosis-infected mice. Infect Immun 1998; 66: 169–175.
Altare F et al. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science 1998; 280: 1432–1435.
Stenger S et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science 1998; 282: 121–125.
Denis O et al. Vaccination with plasmid DNA encoding mycobacterial antigen 85A stimulates a CD4+ and CD8+ T-cell epitopic repertoire broader than that stimulated by Mycobacterium tuberculosis H37Rv infection. Infect Immun 1998; 66: 1527–1533.
Huygen K et al. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med 1996; 2: 893–898.
Lozes E et al. Immunogenicity and efficacy of a tuberculosis DNA vaccine encoding the components of the secreted antigen 85 complex. Vaccine 1997; 15: 830–833.
Kamath AT et al. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect Immun 1999; 67: 1702–1707.
Tascon RE et al. Vaccination against tuberculosis by DNA injection. Nat Med 1996; 2: 888–892.
Lowrie DB et al. Protection against tuberculosis by a plasmid DNA vaccine. Vaccine 1997; 15: 834–838.
Tanghe A et al. Immunogenicity and protective efficacy of tuberculosis DNA vaccines encoding putative phosphate transport receptors. J Immunol 1999; 162: 1113–1119.
Turner J et al. Effective preexposure tuberculosis vaccines fail to protect when they are given in an immunotherapeutic mode. Infect Immun 2000; 68: 1706–1709.
Repique CJ, Li A, Collins FM, Morris SL . DNA immunization in a mouse model of latent tuberculosis: effect of DNA vaccination on reactivation of disease and on reinfection with a secondary challenge. Infect Immun 2002; 70: 3318–3323.
Lowrie DB et al. Therapy of tuberculosis in mice by DNA vaccination. Nature 1999; 400: 269–271.
Lowrie DB, Silva CL . Enhancement of immunocompetence in tuberculosis by DNA vaccination. Vaccine 2000; 18: 1712–1716.
Ha SJ et al. Engineering N-glycosylation mutations in IL-12 enhances sustained cytotoxic T lymphocyte responses for DNA immunization. Nat Biotechnol 2002; 20: 381–386.
McCune RM, Feldmann FM, Lambert HP, McDermott W . Microbial persistence. I. The capacity of tubercle bacilli to survive sterilization in mouse tissues. J Exp Med 1966; 123: 445–468.
Cooper AM et al. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med 1993; 178: 2243–2247.
Flynn JL et al. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med 1993; 178: 2249–2254.
Ottenhof TH, Kumararatne D, Casanova JL . Novel human immunodeficiencies reveal the essential role of type-1 cytokines in immunity to intracellular bacteria. Immunol Today 1998; 19: 491–494.
Dieli F et al. Change of Th0 to Th1 cell-cytokine profile following tuberculosis chemotherapy. Scand J Immunol 2000; 52: 96–102.
Wilkinson RJ et al. Peptide-specific T cell response to Mycobacterium tuberculosis: clinical spectrum, compartmentalization, and effect of chemotherapy. J Infect Dis 1998; 178: 760–768.
Krieg AM . Immune effects and mechanisms of action of CpG motifs. Vaccine 2000; 19: 618–622.
Zhang M et al. T-cell cytokine responses in human infection with Mycobacterium tuberculosis. Infect Immun 1995; 63: 3231–3234.
Song CH et al. Depressed interleukin-12 (IL-12), but not IL-18, production in response to a 30- or 32-kilodalton mycobacterial antigen in patients with active pulmonary tuberculosis. Infect Immun 2000; 68: 4477–4484.
Huygen K et al. Specific lymphoproliferation, gamma interferon production, and serum immunoglobulin G directed against a purified 32 kDa mycobacterial protein antigen (P32) in patients with active tuberculosis. Scand J Immunol 1988; 27: 187–194.
Torres M et al. Cytokine profiles for peripheral blood lymphocytes from patients with active pulmonary tuberculosis and healthy household contacts in response to the 30-kilodalton antigen of Mycobacterium tuberculosis. Infect Immun 1998; 66: 176–180.
Skeiky YA et al. T cell expression cloning of a Mycobacterium tuberculosis gene encoding a protective antigen associated with the early control of infection. J Immunol 2000; 165: 7140–7149.
Pathan AA et al. Direct ex vivo analysis of antigen-specific IFN-γ-secreting CD4 T cells in Mycobacterium tuberculosis-infected individuals: associations with clinical disease state and effect of treatment. J Immunol 2001; 167: 5217–5225.
Cooper AM et al. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology 1995; 84: 423–432.
Silva RA, Florido M, Appelberg R . Interleukin-12 primes CD4+ T cells for interferon-gamma production and protective immunity during Mycobacterium avium infection. Immunology 2001; 103: 368–374.
Cooper AM et al. Mice lacking bioactive IL-12 can generate protective antigen-specific cellular responses to mycobacterial infection only if the IL-12 p40 subunit is present. J Immunol 2002; 138: 1322–1327.
Cooper AM, Magram J, Ferrante J, Orme IM . Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J Exp Med 1997; 186: 39–45.
Holland SM, Dorman SE . Interleukin-12 (IL-12) treatment in interferon gamma refractory pulmonary M. abscessus infection: clinical response and immunologic reconstitution. J Invest Med 1998; 46(suppl): 218A.
Doherty TM, Sher A . IL-12 promotes drug-induced clearance of Mycobacterium avium infection in mice. J Immunol 1998; 160: 5428–5435.
Lee SW, Cho JH, Sung YC . Optimal induction of hepatitis C virus envelope-specific immunity by bicistronic plasmid DNA inoculation with the granulocyte-macrophage colony-stimulating factor gene. J Virol 1998; 72: 8430–8436.
Acknowledgements
This work was supported by the grant of National Research Lab Program of the National S&T Program from Ministry of S&T (M1-0001-00-0089 and M10204000060-02J0000-05510), the grant of Vaccine Innovation Program from the Sequella Foundation, Rockville, MD, and the grants from Genexine Co., Ltd. and Daewoong Co., Ltd.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ha, SJ., Jeon, BY., Kim, SC. et al. Therapeutic effect of DNA vaccines combined with chemotherapy in a latent infection model after aerosol infection of mice with Mycobacterium tuberculosis. Gene Ther 10, 1592–1599 (2003). https://doi.org/10.1038/sj.gt.3302057
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302057
Keywords
This article is cited by
-
Influenza viral matrix 1 protein aggravates viral pathogenicity by inducing TLR4-mediated reactive oxygen species production and apoptotic cell death
Cell Death & Disease (2023)
-
Extracellular nucleoprotein exacerbates influenza virus pathogenesis by activating Toll-like receptor 4 and the NLRP3 inflammasome
Cellular & Molecular Immunology (2022)
-
Immunogenicity and therapeutic effects of a Mycobacterium tuberculosis rv2190c DNA vaccine in mice
BMC Immunology (2017)
-
Adjunctive immunotherapy with α-crystallin based DNA vaccination reduces Tuberculosis chemotherapy period in chronically infected mice
Scientific Reports (2013)
-
In vivo action of IL-27: reciprocal regulation of Th17 and Treg cells in collagen-induced arthritis
Experimental & Molecular Medicine (2013)