Abstract
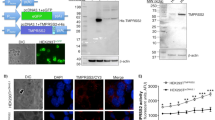
The central role of endoconvertases and HIV-1 protease (HIV-1 PR) in the processing of HIV proproteins makes the design of specific inhibitors important in anti-HIV gene therapy. Accordingly, we tested native α1 antitrypsin (α1AT) delivered by a recombinant simian virus-40-based vector, SV(AT), as an inhibitor of HIV-1 proprotein maturation. Cell lines and primary human lymphocytes were transduced with SV(AT) without selection and detectable toxicity. Expression of α1AT was confirmed by Northern blotting, immunoprecipitation and immunostaining. SV(AT)-transduced cells showed no evidence of HIV-1-related cytopathic effects when challenged with high doses of HIV-1NL4-3. As measured by HIV-1 p24 assay, SV(AT)-transduced cells were protected from HIV-1NL4-3 at challenge dose of 40 000 TCID50 (MOI = 0.04). In addition, peripheral blood lymphocytes treated with SV(AT) were protected from HIV doses challenge up to 40 000 TCID50 (MOI = 0.04). By Western blot analyses, the delivered α1AT inhibited cellular processing of gp160 to gp120 and decreased HIV-1 virion gp120. SV(AT) inhibited processing of p55Gag as well. Furthermore, high levels of uncleaved p55Gag protein were detected in HIV virus particles recovered from SV(AT)-transduced cells lines and primary lymphocytes. Thus, delivering α1AT using SV(AT) to human lymphocytes strongly inhibits replication of HIV-1, most likely by inhibiting the activities both of the cellular serine proteases involved in processing gp160 and of the aspartyl protease, HIV-1 PR, which cleaves p55Gag. α1AT delivered by SV(AT) may represent a novel and effective strategy for gene therapy to interfere with HIV replication, by blocking a stage in the virus replicative cycle that has until now been inaccessible to gene therapeutic intervention.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Trono D, Feinberg MB, Baltimore D . HIV-1 Gag mutants can dominantly interfere with the replication of the wild-type virus. Cell 1989; 59: 113–120.
Aguilar-Cordova E et al. Inhibition of HIV-1 by a double transdominant fusion gene. Gene Ther 1995; 2: 181–186.
Malim MH, Cullen BR . HIV-1 structural gene expression requires the binding of multiple Rev monomers to the viral RRE: implications for HIV-1 latency. Cell 1991;65: 241–248.
Pearson L et al. A transdominant tat mutant that inhibits tat-induced gene expression from the human immunodeficiency virus long terminal repeat. Proc Natl Acad Sci USA 1991; 87: 5079–5083.
Babe LM, Rose J, Craik CS . Trans-dominant inhibitory human immunodeficiency virus type 1 protease monomers prevent protease activation and virion maturation. Proc Natl Acad Sci USA 1990; 92: 10 069–10 073.
Lee TC et al. Overexpression of RRE-derived sequences inhibits HIV-1 replication in CEM cells. New Biol 1992; 4: 66–74.
Sullenger BA et al. Overexpression of TAR sequences renders cells resistant to human immunodeficiency virus replication. Cell 1990; 63: 601–608.
Sczakiel G, Pawlita M . Inhibition of human immunodeficiency virus type 1 replication in human T cells stably expressing antisense RNA. J Virol 1991; 65: 468–472.
Duan L et al. Potent inhibition of human immunodeficiency virus type 1 replication by an intracellular anti-Rev single-chain antibody. Proc Natl Acad Sci USA 1994; 91: 5075–5079.
Marasco WA, Haseltine WA, Chen SY . Design, intracellular expression, and activity of a human anti-human immunodeficiency virus type 1 gp120 single-chain antibody. Proc Natl Acad Sci USA 1993; 90: 7889–7893.
Mhashilkar AM et al. Inhibition of human immunodeficiency virus type 1 replication in vitro in acutely and persistently infected human CD4+ mononuclear cells expressing murine and humanized anti-human immunodeficiency virus type 1 Tat single-chain variable fragment intrabodies. Hum Gene Ther 1999; 10: 1453–1467.
Sarver N et al. Ribozymes as potential anti-HIV-1 therapeutic agents. Science 1990; 247: 1222–1225.
Ojwang JO et al. Inhibition of human immunodeficiency virus type 1 expression by a hairpin ribozyme. Proc Natl Acad Sci USA 1992; 89: 10 802–10 806.
San Jose E, Munoz-Fernandez MA, Alarcon B . Retroviral vector-mediated expression in primary human T cells of an endoplasmic reticulum-retained CD4 chimera inhibits human immunodeficiency virus type-1 replication. Hum Gene Ther 1998; 9: 1345–1357.
Allan JS et al. Major glycoprotein antigens that induce antibodies in AIDS patients are encoded by HTLV-III. Science 1985; 228: 1091–1094.
McCune JM et al. Endoproteolytic cleavage of gp160 is required for the activation of human immunodeficiency virus. Cell 1988; 53: 55–67.
Kowalski M et al. Functional regions of the envelope glycoprotein of human immunodeficiency virus type 1. Science 1987; 237: 1351–1355.
Hallenberger S et al. The role of eukaryotic subtilisin-like endoproteases for the activation of human immunodeficiency virus glycoproteins in natural host cells. J Virol 1997; 71: 1036–1045.
Hallenberger S et al. Inhibition of furin-mediated cleavage activation of HIV-1 glycoprotein gp160. Nature 1992; 360: 358–361.
Moulard M, Decroly E . Maturation of HIV envelope glycoprotein precursors by cellular endoproteases. Biochim Biophys Acta 2000; 1469: 121–132.
Kohl NE et al. Active human immunodeficiency virus protease is required for viral infectivity. Proc Natl Acad Sci USA 1988; 85: 4686–4690.
Gottlinger HG, Sodroski JG, Haseltine WA . Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci USA 1989; 86: 5781–5785.
Travis J, Salvesen GS . Human plasma proteinase inhibitors. Annu Rev Biochem 1983; 52: 655–709.
Strayer DS . SV40 as an effective gene transfer vector in vivo. J Biol Chem 1996; 271: 24 741–24 746.
Strayer DS et al. Use of SV40-based vectors to transduce foreign genes to normal human peripheral blood mononuclear cells. Gene Ther 1997; 4: 219–225.
Strayer DS, Milano J . SV40 mediates stable gene transfer in vivo. Gene Ther 1996; 3: 581–587.
Strayer DS . Gene therapy using SV40-derived vectors: what does the future hold? J Cell Physiol 1999; 181: 375–384.
BouHamdan M et al. Inhibition of HIV-1 by an anti-integrase single-chain variable fragment (SFv): delivery by SV40 provides durable protection against HIV-1 and does not require selection. Gene Ther 1999; 6: 660–666.
Beatty K, Bieth J, Travis J . Kinetics of association of serine proteinases with native and oxidized alpha-1-proteinase inhibitor and alpha-1-antichymotrypsin. J Biol Chem 1980; 255: 3931–3934.
Matheson NR, van Halbeek H, Travis J . Evidence for a tetrahedral intermediate complex during serpin- proteinase interactions. J Biol Chem 1991; 266: 13 489–13 491.
Dalgleish AG et al. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature 1984; 312: 763–767.
Franzusoff A et al. Biochemical and genetic definition of the cellular protease required for HIV-1 gp160 processing. J Biol Chem 1995; 270: 3154–3159.
Anderson ED et al. Inhibition of HIV-1 gp160-dependent membrane fusion by a furin-directed alpha 1-antitrypsin variant. J Biol Chem 1993; 268: 24 887–24 891.
Jean F et al. alpha1-antitrypsin Portland, a bioengineered serpin highly selective for furin: application as an antipathogenic agent. Proc Natl Acad Sci USA 1998; 95: 7293–7298.
Bahbouhi B et al. Effect of alpha-1 antitrypsin portland variant (alpha1-PDX) on HIV-1 replication. Biochem J 2000; 352: 91–98.
Shapiro L, Pott GB, Ralston AH . Alpha-1-antitrypsin inhibits human immunodeficiency virus type 1. FASEB J 2001; 15: 115–122.
Guo HG et al. Characterization of an HIV-1 point mutant blocked in envelope glycoprotein cleavage. Virology 1990; 174: 217–224.
Moulard M et al. Processing and routage of HIV glycoproteins by furin to the cell surface. Virus Res 1999; 60: 55–65.
Decroly E et al. The convertases furin and PC1 can both cleave the human immunodeficiency virus (HIV)-1 envelope glycoprotein gp160 into gp120 (HIV-1 SU) and gp41 (HIV-I TM). J Biol Chem 1994; 269: 12240–12247.
Ho DD et al. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 1995; 373: 123–126.
Gulnik SV et al. Kinetic characterization and cross-resistance patterns of HIV-1 protease mutants selected under drug pressure. Biochemistry 1995; 34: 9282–9287.
Condra JH et al. In vivo emergence of HIV-1 variants resistant to multiple protease inhibitors. Nature 1995; 374: 569–571.
Scharpe S et al. alpha-1-Anti-trypsin, an inhibitor of renin. Biochem J 1976; 153: 505–507.
Jayan GC et al. SV40-derived vectors provide effective transgene expression and inhibition of HIV-1 using, constitutive, conditional and pol III promoters. Gene Ther 2001; 8: 1033–1042.
Zern MA et al. A novel SV40-based vector successfully transduces and expresses an alpha 1-antitrypsin ribozyme in a human hepatoma-derived cell line. Gene Ther 1999; 6: 114–120.
Kondo R, Feitelson MA, Strayer DS . Use of SV40 to immunize against hepatitis B surface antigen: implications for the use of SV40 for gene transduction and its use as an immunizing agent. Gene Ther 1998; 5: 575–582.
Sauter BV et al. A replication-deficient rSV40 mediates liver-directed gene transfer and a long-term amelioration of jaundice in gunn rats. Gastroenterology 2000; 119: 1348–1357.
Strayer DS et al. Titering replication-defective virus for use in gene transfer. Biotechniques 1997; 22: 447–450.
Johnson V, Byington R . Quantitative Assays for Virus Infectivity. Basic Virologic Tech 1988; 71–76.
Acknowledgements
The authors appreciate the technical support of Mr Charles Ko and Ms Maria Lamothe. This work was supported by NIH grants A148244 and RR13156.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cordelier, P., Zern, M. & Strayer, D. HIV-1 proprotein processing as a target for gene therapy. Gene Ther 10, 467–477 (2003). https://doi.org/10.1038/sj.gt.3301891
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301891
Keywords
This article is cited by
-
Alpha-1-antitrypsin interacts with gp41 to block HIV-1 entry into CD4+ T lymphocytes
BMC Microbiology (2016)
-
HIV-1-Infected Astrocytes and the Microglial Proteome
Journal of Neuroimmune Pharmacology (2008)
-
Gene therapy progress and prospects: Novel gene therapy approaches for AIDS
Gene Therapy (2005)
-
Conditional expression of α1-antitrypsin delivered by recombinant SV40 vectors protects lymphocytes against HIV
Gene Therapy (2003)