Abstract
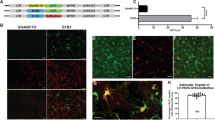
Transplantation of ex vivo gene-corrected autologous cells represents an attractive therapeutic approach for brain diseases. Among the cells of the central nervous system, brain macrophages are promising candidates due to their role in tissue homeostasis and their implication in several neurological diseases. Up to now, gene transfer into macrophages has proven difficult by most currently available gene delivery methods. We describe herein, an efficient transduction of rat bone marrow-derived and brain macrophages with an HIV-1-derived vector containing a central DNA flap and encoding the GFP reporter gene (TRIP-ΔU3-GFP). In primary cultures of macrophages our results show that more than 90% of the cells were transduced by the TRIP vector and that GFP expression remained stable for 1 month without cytopathic effect. In vivo, transplants of transduced macrophages into the striatum of adult rats exhibited long-term expression of GFP up to 3 months. Transduced macrophages were observed around the brain injection site and exhibited the brain macrophage/microglia phenotype. There was no significant sign of astrogliosis around the graft. These results confirm the potential of lentiviral vectors for efficient and stable ex vivo transduction of macrophages. Moreover, transduced autologous macrophages appear as a valuable vehicle for long-term and localized gene expression into the brain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Cortez N., Trejo F., Vergara P., Segovia J. . Primary astrocytes retrovirally transduced with a tyrosine hydroxylase transgene driven by a glial-specific promoter elicit behavioral recovery in experimental parkinsonism J Neurosci Res 2000 59: 39 39
Corti O. et al. A single adenovirus vector mediates doxycycline-controlled expression of tyrosine hydroxylase in brain grafts of human neural progenitors Nat Biotechnol 1999 17: 349 349
Gage F.H., Kawaja M.D., Fisher L.J. . Genetically modified cells: applications for intracerebral grafting Trends Neurosci 14 1991: 328 328
Martinez-Serrano A. . Immortalized neural progenitor cells for CNS gene transfer and repair Trends Neurosci 1997 20: 530 530
Mulligan R. . The basis science of gene therapy Science 1993 260: 926 926
Fisher L.J. et al. Survival and function of intrastriatally grafted primary fibroblasts genetically modified to produce L-dopa Neuron 1991 6: 371 371
Nakao N. et al. Overexpressing Cu/Zn superoxide dismutase enhances survival of transplanted neurons in a rat model of Parkinson's disease Nat Med 1995 1: 226 226
Barkats M. et al. Intrastriatal grafts of embryonic mesencephalic rat neurons genetically modified using an adenovirus encoding human Cu/Zn superoxide dismutase Neuroscience 1997 78: 703 703
Krall W.J. et al. Cells expressing human glucocerebrosidase from a retroviral vector repopulate macrophages and central nervous system microglia after murine bone marrow transplantation Blood 1994 83: 2737 2737
Steven A. . Origin of macrophages in the central nervous tissue J Neurol Sci 1993 118: 117 117
Krivit W., Shapiro E.G., Lackman L.A. . Microglia: the effector cell for reconstitution of the central nervous system following bone marrow transplantation for lysosomal and peroxisomal storage diseases Cell Transplant 1995 4: 385 385
Walker S.U., Dobrennis K., Huang M. . Bone marrow transplantation corrects the enzyme defect in numerous of the central nervous sytem in a lysosomal storage disease Proc Natl Acad Sci USA 1994 91: 2970 2970
Walker S.U., Dobrennis K., Huang M. . Bone marrow transplantation corrects the enzyme defect in numerous of the central nervous sytem in a lysosomal storage disease
Yeager AM, Hart C, Pardoll DM. Repopulation by donnor-derived macrophages in the murine central nervous system (CNS) after congenic bone marrow transplantation (BMT): a quantitative study. Blood 1992; 80 (Suppl. 1): Abstr. 269a Proc Nal Acad Sci USA 1998 95: 14944 14944
Byrnes A.P., Wood M.J., Charlton H.M. . Role of T cells in inflammation caused by adenovirus vectors in the brain Gene Therapy 1996 3: 644 644
Ugolini G. . Transneuronal transfer of herpes simplex virus type 1 (HSV 1) from mixed limb nerves to the CNS: I. Sequence of transfer from sensory, motor, and sympathetic nerve fibres to the spinal cord J Comp Neurol 1992 326: 527 527
Costantini L.C. et al. Gene transfer to the nigrostriatal system by hybrid herpes simplex virus/adeno-associated virus amplicon vectors (published erratum appears in Hum Gene Ther 2000; 11: 981) Hum Gene Ther 1999 10: 2481 2481
Costantini L.C., Bakowska J.C., Breakefield X.O., Isacson O. . Gene therapy in the CNS Gene Therapy 2000 7: 93 93
Baszler T.V., Zachary J.F. . Murine retroviral neurovirulence correlates with an enhanced ability of virus to infect selectively, replicate in, and activate resident microglial cells (published erratum appears in Am J Pathol 1991; 138: 1058) Am J Pathol 1991 138: 655 655
Zachary J.F., Baszler T.V., French R.A., Kelley K.W. . Mouse Moloney leukemia virus infects microglia, but not neurons even though it induces motor neuron disease Molec Psych 1997 2: 104 104
Naldini L. . Lentiviruses as gene transfer agents for delivery to non-dividing cells Curr Opin Biotechnol 1998 9: 457 457
Naldini L. et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector Science 1996 272: 263 263
Blomer U. et al. Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector J Virol 1997 71: 6641 6641
Zufferey R. et al. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery J Virol 1998 72: 9873 9873
Zennou V. et al. HIV-1 genome nuclear import is mediated by a central DNA flap Cell 2000 101: 173 173
Zennou V. et al. HIV-1 genome nuclear import is mediated by a central DNA flap
Sirven A et al. The human immunodeficiency virus type 1 central DNA flap is a crucial determinant for lentiviral vector nuclear import and gene transduction of human hematopoietic stem cells Blood; 2000; 96: 4103–4110 Molec Ther 2001 3: 438 438
Yee J., Laporte P., Bouic K. . A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepathocytes Proc Natl Acad Sci USA 1994 91: 9564 9564
Corbeau P., Kraus G., Wong-Staal F. . Transduction of human macrophages using a stable HIV-1/HIV-2-derived gene delivery system Gene Therapy 1998 5: 99 99
Schroers R. et al. Transduction of human PBMC-derived dendritic cells and macrophages by an HIV-1-based lentiviral vector system Molec Ther J Am Soc Gene Ther 2000 1: 171 171
Wada R., Proia R.L. . Microglia activation precedes acute neurodegeneration in Sandhoff disease and is suppressed by bone marrow transplantation Proc Natl Acad Sci USA 2000 97: 10954 10954
Persidsky Y., Zheng J., Miller D., Gendelman H.E. . Mononuclear phagocytes mediate blood–brain barrier compromise and neuronal injury during HIV-1-associated dementia J Leuk Biol 2000 68: 413 413
Kennedy D.W., Abkowitz J.L. . Kinetics of central nervous system microglial and macrophage engraftment: analysis using a transgenic bone marrow transplantation model Blood 1997 90: 986 986
Bachoud-Levi A.C. et al. Motor and cognitive improvements in patients with Huntington's disease after neural transplantation Lancet 2000 356: 1975 1975
Kordower J.H., Bloch J. . Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease Science 2000 290: 767 767
Kordower J.H., Bloch J. . Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease
Ridet JL, Serguera C, Zennou V. Primary astrocytes: a potent and safe vehicle for ex vivo gene transfer to the CN. (Submitted for publication) Eur J Neurosci 1996 8: 1725 1725
Tushinski R.J. et al. Survival of mononuclear phagocytes depends on a lineage-specific growth factor that the differentiated cells selectively destroy Cell 1982 28: 71 71
Stanley E.R. . Factors regulating macrophage production and growth J Biol Chem 1977 252: 4305 4305
Altmeyer R., Mordelet E., Girard M., Vidal C. . Expression and detection of macrophage-tropic HIV-1 gp120 in the brain using conformation-dependent antibodies Virology 1999 259: 314 314
Mordelet E., Altmeyer R., Girard M., Vidal C. . HIV-1 gp120: expression and subcellular localisation in macrophages/microglia in vitro and in vivo in the rat brain J Neurovirol 1998 4: 360 360
Barclay A.N. . Different reticular elements in rat lymphoid tissue identified by localization of Ia, TH-1 and MRC OX 2 antigens Immunology 1981 44: 727 727
Dijkstra C.D. . The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3 Immunology 1985 54: 589 589
Balasingam V., Collier B., Yong V.W. . Attenuation of astroglial reactivity by IL-10 Soc Neurosci Abstr 1995 21: 304 304
Acknowledgements
E Mordelet was supported by fellowships from the Pasteur-Weizmann Foundation and from SIDACTION. This work was supported by ANRS and AFM. We thank R Hellio and P Roux for assistance at the laser confocal microscope which was purchased with the donation from Marcel and Liliane Pollack.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mordelet, E., Kissa, K., Calvo, CF. et al. Brain engraftment of autologous macrophages transduced with a lentiviral flap vector: an approach to complement brain dysfunctions. Gene Ther 9, 46–52 (2002). https://doi.org/10.1038/sj.gt.3301591
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301591
Keywords
This article is cited by
-
Rational targeting for prion therapeutics
Nature Reviews Neuroscience (2005)
-
Newborn liver gene transfer by an HIV-2-based lentiviral vector
Gene Therapy (2005)
-
Selective gene expression in brain microglia mediated via adeno-associated virus type 2 and type 5 vectors
Gene Therapy (2003)
-
Viral vectors for gene delivery to the nervous system
Nature Reviews Neuroscience (2003)