Abstract
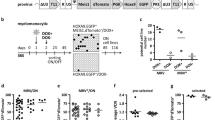
Various protocols have been described to optimize gene transfer into hematopoietic cells. However, most of these methods do not specify whether they are associated with an improved transduction of the more primitive stem/progenitor cells, the best candidates for long-term engraftment. The majority of these primitive cells remains in quiescence because of the negative control of TGF-β1, effective on these cells at low concentrations (10 pg/ml). In this study, CD34+ cells were activated by a 10 h pretreatment with anti-TGF-β1 followed by four successive retroviral supernatant incubations of 6 h each. After 12 h (two incubations), a significant increase in TGF-β1 mRNA in CD34+ cells was observed. We wondered whether neo-synthesized autocrine TGF-β1 could induce reversion to quiescence of the more primitive CD34+ cells transduced after one cell cycle. This would prevent their subsequent detection in a classic clonal assay. Using the HPP-Q assay comparing a rapid mixed colony assay with or without anti-TGF-β1, we indeed observed, that in clonal growth conditions the more primitive transduced cells were activated and detectable only with anti-TGF-β1. Therefore, this assay represents not only a rapid means to detect quiescent multipotent stem/progenitor cells but also a necessary step for the detection of the more primitive transduced cells which have returned to quiescence after retroviral induction of TGF-β1 secretion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Roberts AB et al. Transforming growth factor-β: multifunctional regulator of differentiation and development Philos Trans R Soc Lond B Biol Sci 1990 327: 145–154
Keller JR et al. Transforming growth factor-β: a bidirectional regulator of hematopoietic cell growth Int J Cell Cloning 1992 10: 2–11
Ohta M et al. Two forms of transforming growth factor-β distinguished by multipotential haematopoietic progenitor cells Nature 1987 329: 539–541
Fortunel NO, Hatzfeld A, Hatzfeld JA . Transforming growth factor-β: pleiotropic role in the regulation of hematopoiesis Blood 2000 96: 2022–2036
Hatzfeld J et al. Release of early human hematopoietic progenitors from quiescence by antisense transforming growth factor-β1 or Rb oligonucleotides J Exp Med 1991 174: 925–929
Cardoso AA et al. Release from quiescence of CD34+ CD38− human umbilical cord blood cells reveals their potentiality to engraft adults Proc Natl Acad Sci USA 1993 90: 8707–8711
Fortunel N et al. High proliferative potential-quiescent cells: a working model to study primitive quiescent hematopoietic cells J Cell Sci 1998 111: 1867–1875
Fortunel N et al. Release from quiescence of primitive human hematopoietic stem/progenitor cells by blocking their cell-surface TGF-β type II receptor in a short-term in vitro assay Stem Cells 2000 18: 102–111
Dao MA, Taylor N, Nolta JA . Reduction in levels of the cyclin-dependent kinase inhibitor p27(Kip-1) coupled with transforming growth factor-β neutralization induces cell-cycle entry and increases retroviral transduction of primitive human hematopoietic cells Proc Natl Acad Sci USA 1998 95: 13006–13011
Fortunel N et al. Specific dose-response effects of TGF-β1 on developmentally distinct hematopoietic stem/progenitor cells from human umbilical cord blood Hematology J 2000 1: 133–142
Hatzfeld A et al. Increased stable retroviral gene transfer in early hematopoietic progenitors released from quiescence Hum Gene Ther 1996 7: 207–213
Steinman RA et al. Regulation of p21(WAF1) expression during normal myeloid differentiation Blood 1998 91: 4531–4542
Ducos K et al. P21cip1 mRNA is controlled by endogenous transforming growth factor-β1 in quiescent human hematopoietic stem/progenitor cells J Cell Physiol 2000 184: 80–85
Moritz T, Patel VP, Williams DA . Bone marrow extracellular matrix molecules improve gene transfer into human hematopoietic cells via retroviral vectors J Clin Invest 1994 93: 1451–1457
Moritz T et al. Fibronectin improves transduction of reconstituting hematopoietic stem cells by retroviral vectors: evidence of direct viral binding to chymotryptic carboxy-terminal fragments Blood 1996 88: 855–862
Byun J et al. Analysis of the relative level of gene expression from different retroviral vectors used for gene therapy Gene Therapy 1996 3: 780–788
Krall WJ et al. Increased levels of spliced RNA account for augmented expression from the MFG retroviral vector in hematopoietic cells Gene Therapy 1996 3: 37–48
Forestell SP, Böhnlein E, Rigg RJ . Retroviral end-point titer is not predictive of gene transfer efficiency: implications for vector production Gene Therapy 1995 2: 723–730
Panterne B et al. IL-3, GM-CSF and CSF-1 modulate c-fms mRNA more rapidly in human early monocytic progenitors than in mature or transformed monocytic cells J Cell Sci 1996 109: 1795–1801
Villacres-Eriksson M et al. Involvement of interleukin-2 and interferon-gamma in the immune response induced by influenza virus iscoms Scand J Immunol 1992 36: 421–426
Gessani S et al. Induction of beta interferon by human immunodeficiency virus type 1 and its gp120 protein in human monocytes–macrophages: role of beta interferon in restriction of virus replication J Virol 1994 68: 1983–1986
Sansilvestri P et al. Early CD34high cells can be separated into KIThigh cells in which transforming growth factor-β (TGF-β) downmodulates c-kit and KITlow cells in which anti-TGF-β upmodulates c-kit Blood 1995 86: 1729–1735
Hanenberg H et al. Colocalization of retrovirus and target cells on specific fibronectin fragments increases genetic transduction of mammalian cells Nature Med 1996 2: 876–882
Kawashima I et al. CD34+ human marrow cells that express low levels of Kit protein are enriched for long-term marrow-engrafting cells Blood 1996 87: 4136–4142
Batard P et al. TGF-β1 maintains hematopoietic immaturity by a reversible negative control of cell cycle and by CD34 antigen up-modulation J Cell Sci 2000 113: 383–390
Dick JE et al. Assay of human stem cells by repopulation of NOD/SCID mice Stem Cells 1997 15 (Suppl. 1): 199–207
Brossard Y et al. Collection of placental blood with a view to hemopoietic reconstitution Nouv Rev Fr Hématol 1990 32: 427–429
Angerer LM, Cox KH, Angerer RC . Demonstration of tissue-specific gene expression by in situ hybridization Meth Enzymol 1987 152: 649–661
Bernaudin JF et al. Demonstration by in situ hybridization of dissimilar IL-1 beta gene expression in human alveolar macrophages and blood monocytes in response to lipopolysaccharide J Immunol 1988 140: 3822–3829
MacGregor GR et al. Use of E. coli LacZ (β-galactosidase) as a reporter gene Meth Mol Biol 1991 7: 217–235
Acknowledgements
We thank Dr RC Mulligan for providing the MFG-nlsLacZ A7–21 producer clone. We thank Dr C Paillissé for her assistance in statistical computations. This study was supported by the Centre National de la Recherche Scientifique (CNRS), the Agence Nationale de la Recherche sur le Sida (ANRS), the European contract No. BIO4-CT96–0646. KD and NF have been recipients of fellowships from the Ministère de l’Éducation Nationale, de l'Enseignement Supérieur et de la Recherche (MENESR) and from the Association pour la Recherche sur le Cancer (ARC). SK was supported by the Direction des Recherches Etudes et Techniques (DRET).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ducos, K., Hatzfeld, A., Héron, A. et al. The high proliferative potential-quiescent (HPP-Q) cell assay allows an optimized evaluation of gene transfer efficiency into primitive hematopoietic stem/progenitor cells. Gene Ther 7, 1790–1794 (2000). https://doi.org/10.1038/sj.gt.3301304
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301304