Abstract
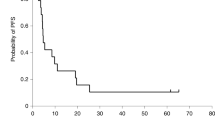
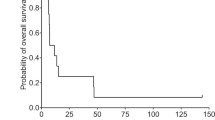
Herpes simplex virus (HSV)-mediated delivery of the HSV thymidine kinase (tk) gene to tumor cells in combination with ganciclovir (GCV) administration may provide an effective suicide gene therapy for destruction of malignant glioblastomas. However, because HSV is a highly cytotoxic agent, gene expression from the virus is short-lived which may limit the effectiveness of HSVtk/GCV therapy. Using different replication-defective HSVtk gene vectors, we compared HSV vector backgrounds for their cytotoxic activity on infection of 9L gliosarcoma cells in culture and brain tumors in rats and evaluated the impact of vector toxicity on the effectiveness of tk/GCV-mediated suicide gene therapy. As reported previously for other cell lines, a vector deleted for both copies of the immediate–early (IE) gene ICP4 (SOZ.1) was highly toxic for 9L cells in culture while a vector deleted in addition for the ICP22 and ICP27 IE genes (T.1) reduced or arrested 9L cell proliferation with more limited cell killing. Nevertheless, both vectors supported widespread killing of uninfected cells in the presence of GCV following low multiplicity infections, indicating that vector cytotoxicity did not preempt the production of vector-encoded TK enzyme necessary for the killing of uninfected cells by the HSV-tk/GCV bystander effect. Although an SOZ.1-related vector (SHZ.2) caused tumor cell necrosis in vivo, injection of SHZ.2 at multiple coordinates thoughout the tumor followed by GCV administration failed to prolong markedly the survival of tumor- bearing rats. In contrast, a single injection of T.1 produced a life-extending response to GCV. These results indicate that vector cytotoxicity can limit the efficacy of HSV-tk/GCV treatment in vivo, which may be due to premature termination of tk gene expression with attendant abortion of the bystander effect.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Arcicasa M et al. Results of three consecutive combined treatments for malignant gliomas. Ten-year experience at a single institution Am J Clin Oncol 1994 17: 437–443
Mahaley MS Jr et al. National survey of patterns of care for brain-tumor patients J Neurosurg 1989 71: 826–836
Ushio Y . Treatment of gliomas in adults Curr Opin Oncol 1991 3: 467–475
Chang SM, Prados MD . Chemotherapy for gliomas Curr Opin Oncol 1995 7: 207–213
Kaye AH, Laidlaw JD . Chemotherapy for gliomas Curr Opin Neurol Neurosurg 1992 5: 526–533
Salcman M . Epidemiology and factors affecting survival. In: Appuzzo MLJ (ed) Malignant Cerebral Glioma American Association of Neurological Surgeons, AANS Publications Committee: Park Ridge, IL 1990 95–110
Hishii M et al. Human glioma-derived interleukin-10 inhibits antitumor immune responses in vitro Neurosurgery 1995 37: 1160–1167
Ruffini PA et al. Factors, including transforming growth factor beta, released in the glioblastoma residual cavity, impair activity of adherent lymphokine-activated killer cells Cancer Immunol Immunother 1993 36: 409–416
Barba D et al. Development of antitumor immunity for following thymidine kinase-mediated killing of experimental brain tumors Proc Natl Acad Sci USA 1994 91: 4348–4352
Chen SH et al. Gene therapy for brain tumors: regression of experimental gliomas by adenovirus-mediated gene transfer in vivo Proc Natl Acad Sci USA 1994 91: 3054–3057
Perez-Cruet M et al. Adenovirus-mediated gene therapy of experimental gliomas J Neurosci Res 1994 39: 506–511
Ram Z et al. Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells Nature Med 1997 3: 1354–1361
Freeman SM et al. The ‘bystander effect’: tumor regression when a fraction of the tumor mass is genetically modified Cancer Res 1993 53: 5274–5283
Fink DJ et al. In vivo expression of β-galactosidase in hippocampal neurons by HSV-mediated gene transfer Hum Gene Ther 1992 3: 11–19
DeLuca NA, McCarthy AM, Schaffer PA . Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate–early regulatory protein ICP4 J Virol 1985 56: 558–570
DeLuca NA, Schaffer PA . Activation of immediate–early, early, and late promoters by temperature-sensitive and wild-type forms of herpes simplex virus type 1 protein ICP4 Mol Cell Biol 1985 5: 1997–2008
Krisky DM et al. Development of herpes simplex virus replication defective multigene vectors for combination gene therapy applications Gene Therapy 1998 5: 1517–1530
Krisky DM et al. Deletion of multiple immediate–early genes from herpes simplex virus reduces cytotoxicity and permits long-term gene expression in neurons Gene Therapy 1998 5: 1593–1603
Wu N et al. Prolonged gene expression and cell survival after infection by a herpes simplex virus mutant defective in the immediate–early genes encoding ICP4, ICP27, and ICP22 J Virol 1996 70: 6358–6368
Johnson P, Wang M, Friedmann T . Improved cell survival by the reduction of immediate–early gene expression in replication-defective mutants of herpes simplex virus type 1 but not by mutation of the viron host shutoff function J Virol 1994 68: 6347–6362
Marconi P et al. Replication-defective HSV vectors for gene transfer in vivo Proc Natl Acad Sci USA 1996 93: 11319–11320
Samaniego LA . Neiderhiser L, DeLuca NA. Persistence and expression of the herpes simplex virus genome in the absence of immediate–early proteins J Virol 1998 72: 3307–3320
Akkaraju GR et al. Herpes simplex virus vector-mediated dystrophin gene transfer and expression in MDX mouse skeletal muscle J Gene Med 1999 1: 280–289
Huard J et al. Gene transfer to muscle using herpes simplex virus-based vectors Neuromusc Dis 1997 7: 299–313
Read GS, Frenkel N . Herpes simplex virus mutants defective in the virion-associated shutoff of host polypeptide synthesis and exhibiting abnormal synthesis of α (immediate early) viral polypeptides J Virol 1983 46: 498–512
Krisky DM et al. Rapid method for construction of recombinant HSV gene transfer vectors Gene Therapy 1997 4: 1120–1125
Culver K et al. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors Science 1992 256: 1550–1552
Dewey RA et al. Chronic brain inflammation and persistent herpes simplex virus 1 thymidine kinase expression in survivors of syngeneic glioma treated by adenovirus-mediated gene therapy: implications for clinical trials Nature Med 1999 5: 1256–1263
Marconi P et al. Connexin43-enhanced suicide gene therapy using herpesviral vectors Mol Ther 2000 1: 71–81
Krisky D et al. Development of replication-defective herpes simplex virus vectors. In: Robbins P (ed) Methods in Molecular Medicine, Gene Therapy Protocols Humana Press: New Jersey 1996 79–102
Gage PJ et al. A cell free recombination system for site-specific integration of multigenic shuttle plasmids into the herpes simplex virus type 1 genome J Virol 1992 66: 5509–5515
Mosmann T . Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays J Immunol Methods 1983 65: 55–63
Sanes JR, Rubenstein JL, Nicolas JF . Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos EMBO J 1986 5: 3133–3142
Niranjan A et al. Effective treatment of experimental glioblastoma by HSV vector-mediated TNFa and HSV-tk gene transfer in combination with radiosurgery and ganciclovir administration Mol Ther (in press)
Acknowledgements
We wish to thank Kate Sullivan of the Department of Molecular Genetics and Biochemistry for technical assistance. This work was supported by NIH Grants GM34534 and AR44526 to JCG.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Moriuchi, S., Krisky, D., Marconi, P. et al. HSV vector cytotoxicity is inversely correlated with effective TK/GCV suicide gene therapy of rat gliosarcoma. Gene Ther 7, 1483–1490 (2000). https://doi.org/10.1038/sj.gt.3301265
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301265
Keywords
This article is cited by
-
Evaluation and optimization of the administration of a selectively replicating herpes simplex viral vector to the brain by convection-enhanced delivery
Cancer Gene Therapy (2011)
-
The involvement of nuclear factor-kappa B in cyclooxygenase-2 overexpression in murine colon cancer cells transduced with herpes simplex virus thymidine kinase gene
Cancer Gene Therapy (2006)
-
Viral gene therapy
Clinical and Translational Oncology (2006)
-
Development and application of replication-incompetent HSV-1-based vectors
Gene Therapy (2005)
-
Herpes simplex virus thymidine kinase gene transduction enhances tumor growth rate and cyclooxygenase-2 expression in murine colon cancer cells
Cancer Gene Therapy (2004)