Abstract
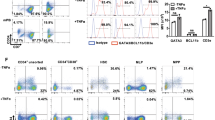
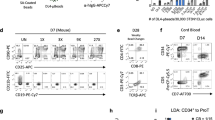
Success of gene therapy for diseases affecting the T cell lineage depends on the thymic repopulation by genetically engineered hematopoietic progenitor cells (HPC). Although it has been shown that retrovirally transduced HPC can repopulate the thymus, little information is available on the effect of the culture protocol. Moreover, for expansion of the number of HPC, cytokine supplemented culture is needed. Here, we transduced purified human umbilical cord blood (CB) CD34+ cells in cultures supplemented with various combinations of the cytokines thrombopoietin (TPO), stem cell factor (SCF), flt3/flk-2 ligand (FL), interleukin-3 (IL-3) and IL-6, and investigated thymus-repopulating ability of gene-marked HPC in vitro. Irrespective of the cytokine cocktail used, transduced CD34+CD38− CB cells, expressing the marker green fluorescent protein (GFP) encoded by the MFG-GFP retrovirus, have both superior proliferative and thymus-repopulating potential compared with transduced CD34+CD38+ CB cells. Effectively transduced GFP+CD34+CD38− HPC, cultured for 3 or 17 days, more readily generated T cells than GFP− HPC from the same culture. The reverse was true in the case of CD34+CD38+ HPC cultures. Finally, our results indicate that the number of GFP+ T cell progenitors actually increased during culture of CD34+CD38− HPC, in a magnitude that is determined by the cytokine cocktail used during culture.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Dunbar CE, Young NS . Gene marking and gene therapy directed at primary hematopoietic cells Curr Opin Hematol 1996 3: 430–437
Moritz T, Keller DC, Williams DA . Human cord blood cells as targets for gene transfer: potential use in genetic therapies of severe combined immunodeficiency disease J Exp Med 1993 178: 529–536
Verhasselt B et al. Retrovirally transduced CD34++ human cord blood cells generate T cells expressing high levels of the retroviral encoded green fluorescent protein marker in vitro Blood 1998 91: 431–440
Miyoshi H et al. Transduction of human CD34(+) cells that mediate long-term engraftment of NOD/SCID mice by HIV vectors Science 1999 283: 682–686
Case SS et al. Stable transduction of quiescent CD34(+)CD38(-) human hematopoietic cells by HIV-1-based lentiviral vectors Proc Natl Acad Sci USA 1999 96: 2988–2993
Gluckman E et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group New Engl J Med 1997 337: 373–381
Brenner MK et al. Gene marking to determine whether autologous marrow infusion restores long-term haemopoiesis in cancer patients Lancet 1993 342: 1134–1137
Dunbar CE et al. Retrovirally marked CD34-enriched peripheral blood and bone marrow cells contribute to long-term engraftment after autologous transplantation Blood 1995 85: 3048–3057
Crystal RG . Transfer of genes to humans: early lessons and obstacles to success Science 1995 270: 404–410
Bordignon C et al. Gene therapy in peripheral blood lymphocytes and bone marrow for ADA-immunodeficient patients Science 1995 270: 470–475
Kohn DB et al. T lymphocytes with a normal ADA gene accumulate after transplantation of transduced autologous umbilical cord blood CD34+ cells in ADA-deficient SCID neonates Nature Med 1998 4: 775–780
Hesdorffer C et al. Phase I trial of retroviral-mediated transfer of the human MDR1 gene as marrow chemoprotection in patients undergoing high-dose chemotherapy and autologous stem-cell transplantation J Clin Oncol 1998 16: 165–172
Stewart AK et al. Engraftment of gene-marked hematopoietic progenitors in myeloma patients after transplant of autologous long-term marrow cultures Hum Gene Ther 1999 10: 1953–1964
Kohn DB et al. Engraftment of gene-modified umbilical cord blood cells in neonates with adenosine deaminase deficiency Nature Med 1995 1: 1017–1023
Douek DC et al. Changes in thymic function with age and during the treatment of HIV infection Nature 1998 396: 690–695
Jamieson BD et al. Generation of functional thymocytes in the human adult Immunity 1999 10: 569–575
Poulin J-F et al. Direct evidence for thymic function in adult humans J Exp Med 1999 190: 479–486
Verhasselt B et al. Thymic repopulation by CD34+ human cord blood cells after expansion in stroma-free culture Blood 1999 94: 3644–3652
Hao QL et al. A functional comparison of CD34+ CD38− cells in cord blood and bone marrow Blood 1995 86: 3745–3753
Shah AJ, Smogorzewska EM, Hannum C, Crooks GM . Flt3 ligand induces proliferation of quiescent human bone marrow CD34+CD38− cells and maintains progenitor cells in vitro Blood 1996 87: 3563–3570
Conneally E, Cashman J, Petzer A, Eaves C . Expansion in vitro of transplantable human cord blood stem cells demonstrated using a quantitative assay of their lympho-myeloid repopulating activity in nonobese diabetic-scid/scid mice Proc Natl Acad Sci USA 1997 94: 9836–9841
Ramsfjell V, Borge OJ, Cui L, Jacobsen SE . Thrombopoietin directly and potently stimulates multilineage growth and progenitor cell expansion from primitive (CD34+ CD38−) human bone marrow progenitor cells: distinct and key interactions with the ligands for c-kit and flt3, and inhibitory effects of TGF-beta and TNF-alpha J Immunol 1997 158: 5169–5177
Agrawal YP et al. Cell-cycle kinetics and VSV-G pseudotyped retrovirus-mediated gene transfer in blood-derived CD34+ cells Exp Hematol 1996 24: 738–747
Hao QL et al. Extended long-term culture reveals a highly quiescent and primitive human hematopoietic progenitor population Blood 1996 88: 3306–3313
Kohn DB et al. Selective accumulation of ADA gene-modified T lymphocytes upon PEG-ADA dosage reduction after gene therapy with transduced CD34+ umbilical cord blood cells Blood 1995 86: (Suppl.1) 1168a (Abstr.)
Bhatia M et al. A newly discovered class of human hematopoietic cells with SCID-repopulating activity Nature Med 1998 4: 1038–1045
Yu M, Poeschla E, Wong Staal F . Progress towards gene therapy for HIV infection Gene Therapy 1994 1: 13–26
Hacein-Bey S et al. γc Gene transfer in the presence of stem cell factor, FLT-3L, interleukin-7 (IL-7), IL-1α, and IL-15 cytokines restores T-cell differentiation from γc(−) X-linked severecombined immunodeficiency hematopoietic progenitor cellsin murine fetal thymic organ cultures Blood 1998 92: 4090–4097
Robin C et al. Identification of human T-lymphoid progenitor cells in CD34+38low and CD34+38+ subsets of human cord blood and bone marrow cells using NOD-SCID fetal thymus organ cultures Br J Haematol 1999 104: 809–819
Acknowledgements
We thank Christian De Boever for artwork, Achiel Moerman, Veronique Debacker, Ilse Swennen and Greet De Smet for animal care, the Departments of Obstetrics, of Cardiac Surgery and of Pathology for the supply of human tissue. This work was supported by grants from the Gezamelijk Overlegde Actie (GOA) University of Ghent; the Fund for Scientific Research – Flanders (Belgium); and the VIB. BV is a research assistant of the Fund for Scientific Research – Flanders (Belgium). EN is a VIB employee.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Verhasselt, B., Naessens, E., De Smedt, M. et al. Efficiency of transgenic T cell generation from gene-marked cultured human CD34+ cord blood cells is determined by their maturity and the cytokines present in the culture medium. Gene Ther 7, 830–836 (2000). https://doi.org/10.1038/sj.gt.3301176
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301176