Abstract
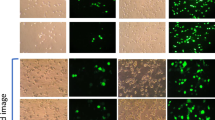
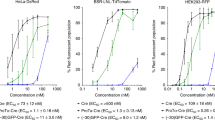
Cationic lipid-based delivery systems such as lipoplexes or stabilized plasmid–lipid particles (SPLP) represent a safer alternative to viral systems for gene therapy applications. We studied the impact of cell cycle status on the efficiency of transfection of human ovarian carcinoma tumor cells using two cationic-lipid based delivery systems. Cells arrested in the G1 phase of the cell cycle by treatment with aphidicolin were compared with an asynchronous dividing population of cells. Treatment of the cells with aphidicolin had no effect on the rate of internalization of the lipid formulated DNA or on the level of gene expression observable in stably transfected cells. However, cells treated with aphidicolin exhibited 20-fold lower reporter gene activity than asynchronous control cells upon incubation with lipoplexes. When cells arrested in the G1 phase were allowed to proceed though the cell cycle in the presence of the lipoplex or SPLP, transgene expression was found to coincide with the transition of cells from the G2/M phase into the G1 phase of the subsequent cell cycle. In addition, higher levels of reporter gene expression were observed when the cells were incubated with lipoplexes or SPLP during, or just before, mitosis. These results suggest that it may be possible to augment cationic lipid-mediated transfection by manipulating the cell cycle status of the target cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Felgner PL et al. Lipofectin: a highly efficient lipid-mediated DNA transfection procedure Proc Natl Acad Sci USA 1987 84: 7413–7417
Felgner PL et al. Improved cationic lipid formulations for in vivo gene therapy Ann NY Acad Sci 1995 772: 126–139
Gao X, Huang L . A novel cationic liposome reagent for efficient transfection of mammalian cells Biochem Biophys Res Commun 1991 179: 280–285
Caplen NJ et al. Liposome-mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis Nature Med 1995 1: 39–46
Nabel GJ et al. Immune response in human melanoma after transfection of an allogenic class I major histocompatibility complex gene with DNA liposome Proc Natl Acad Sci USA 1996 93: 15388–15399
Gill DR et al. A placebo controlled study of liposome-mediated gene transfer to the nasal epithelium of patients with cystic fibrosis Gene Therapy 1997 4: 199–209
Zabner J et al. Cellular and molecular barriers to gene transfer by a cationic lipid J Biol Chem 1995 270: 18997–19007
Wrobel I, Collins D . Fusion of cationic liposomes with mammalian cells occurs after endocytosis Biochim Biophys Acta 1995 1235: 296–304
Xu Y, Szoka FC Jr . Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection Biochemistry 1996 35: 5616–5623
Friend DS, Papahadjopoulos D, Debs RJ . Endocytosis and intracellular processing accompanying transfection mediated by cationic liposomes Biochim Biophys Acta 1996 1278: 41–50
Wilke M et al. Efficacy of a peptide-based gene delivery system depends on mitotic activity Gene Therapy 1996 3: 1133–1142
Nicolau C, Sene C . Liposome-mediated DNA transfer in eukaryotic cells Biochim Biophys Acta 1982 721: 185–190
Fasbender A, Zabner J, Zeiher BG, Welsh MJ . A low rate of cell proliferation and reduced DNA uptake limit cationic lipid-mediated gene transfer to primary cultures of ciliated human airway epithelia Gene Therapy 1997 4: 1173–1180
Jiang C et al. Efficiency of cationic lipid-mediated transfection of polarized and differentiated airway epithelial cells in vitro and in vivo Hum Gene Ther 1998 9: 1531–1542
Bukrinsky MI et al. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells Nature 1993 365: 666–669
Naldini L et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector Science 1996 272: 263–267
von Schwedler U, Kornbluth RS, Trono D . The nuclear localization signal of the matrix protein of human immunodeficiency virus type 1 allows the establishment of infection in macrophages and quiescent T lymphocytes Proc Natl Acad Sci USA 1994 91: 6992–6996
Felgner PL et al. Nomenclature for synthetic gene delivery systems Hum Gene Ther 1997 8: 511–512
Wheeler J et al. Stabilized plasmid-lipid particles: construction and characterization Gene Therapy 1999 (in press)
Ikegami S et al. Aphidicolin prevents mitotic cell division by interfering with the activity of DNA polymerase-alpha Nature 1978 275: 458–460
Wahl AF et al. Properties of two forms of DNA polymerase delta from calf thymus Biochemistry 1986 25: 7821–7827
Tseng W-C et al. Cationic liposomal delivery of plasmid to endothelial cells measured by quantitative flow cytometry Biotechnol Bioeng 1996 50: 548–554
Tseng W-C, Haselton FR, Giorgio TD . Transfection of cationic liposomes using simultaneous single cell measurements of plasmid delivery and transgene expression J Biol Chem 1997 272: 25641–25647
Mulligan RC . The basic science of gene therapy Science 1993 260: 926–932
Verma IM . Gene therapy: hopes, hypes, and hurdles Molec Med 1994 1: 2–3
Greber UF et al. Stepwise dismantling of adenovirus 2 during entry into cells Cell 1993 75: 477–486
Dowty J et al. Plasmid DNA entry into postmitotic nuclei of primary rat myotubules Proc Natl Acad Sci USA 1995 92: 4572–4576
Maniatis T, Fritsch EF, Sambrook J . Molecular Cloning Cold Spring Harbor: New York, 1981, pp 93–94
Liljestrom P, Garoff H . Expression of proteins using Semliki forest virus vectors. In: Ausubel FM et al (eds). Current Protocols in Molecular Biology John Wiley: Canada 1994 (Suppl. 28) 16.20.1–16.20.16
Lechardeur D et al. Metabolic instability of plasmid DNA in the cytosol; a potential barrier to gene transfer Gene Therapy 1999 (in press)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mortimer, I., Tam, P., MacLachlan, I. et al. Cationic lipid-mediated transfection of cells in culture requires mitotic activity. Gene Ther 6, 403–411 (1999). https://doi.org/10.1038/sj.gt.3300837
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3300837
Keywords
This article is cited by
-
Vibropolyfection: coupling polymer-mediated gene delivery to mechanical stimulation to enhance transfection of adherent cells
Journal of Nanobiotechnology (2022)
-
Improved CRISPR/Cas9 gene editing in primary human myoblasts using low confluency cultures on Matrigel
Skeletal Muscle (2021)
-
Entry of PIP3-containing polyplexes into MDCK epithelial cells by local apical-basal polarity reversal
Scientific Reports (2016)
-
Characterization of exogenous DNA mobility in live cells through fluctuation correlation spectroscopy
Scientific Reports (2015)
-
Mammalian synthetic circuits with RNA binding proteins for RNA-only delivery
Nature Biotechnology (2015)