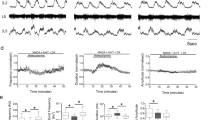
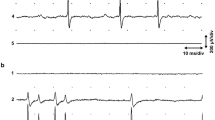
Abstract
Slow muscarinic inhibition may be a powerful influence on membrane properties in the peripheral1–3 and central nervous system4. But the location of the muscarinic receptors in sympathetic ganglia, either on interneurones or on the postganglionic membrane, and the underlying mechanism of the inhibitory response, remains controversial5–7. In mammalian sympathetic ganglia synaptic activation of muscarinic receptors located on inhibitory interneurones was thought to release catecholamines leading to a membrane hyperpolarization called the slow inhibitory postsynaptic potential, or s.-i.p.s.p.8. However, the s.-i.p.s.p. in parasympathetic ganglia3,9,10 and in amphibian sympathetic ganglia1,2 is due to direct monosynaptic activation of muscarinic receptors, accompanied by an increased potassium conductance (but see ref. 11), and is not mediated by catecholamines. The situation is less clear in mammalian sympathetic ganglia and monosynaptic s.-i.p.s.p. observed in other ganglia could be exceptions to the hypothesis. We showed earlier that the s.-i.p.s.p. in rabbit superior cervical ganglia is not affected by catecholamine antagonists12. We now show that the s.-i.p.s.p. in a mammalian sympathetic ganglion is due to the monosynaptic activation of muscarinic receptors, probably by an increase in potassium conductance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Horn, J. P. & Dodd, J. Nature 292, 625–627 (1981).
Dodd, J. & Horn, J. P. J. Physiol., Lond. 334, 271–291 (1983).
Gallagher, J. P., Griffith, W. H. III & Shinnick-Gallagher, P. J. Physiol., Lond. 332, 473–486 (1982).
Krnjevic, K. & Ropert, N. Neuroscience 7, 2165–2183 (1982).
Gallagher, J. P., Shinnick-Gallagher, P., Cole, A. E., Griffith, W. H. & Williams, B. J. Fedn Proc. 39, 3009–3015 (1980).
Libet, B. & Kobayashi, H. J. Neurophysiol. 37, 805–814 (1974).
Weight, F. F. & Padjen, A. Brain Res. 55, 225–228 (1973).
Eccles, R. & Libet, B. J. Physiol., Lond. 157, 484–503 (1961).
Hartzell, H. C., Kuffler, S. W., Stickgold, R. & Yoshikami, D. J. Physiol., Lond. 271, 817–846 (1977).
Griffith, W. H., Gallagher, J. P. & Shinnick-Gallagher, P. Brain Res. 209, 446–451 (1981).
Akasu, T. & Koketsu, K. Jap. J. Physiol. 33, 279–300 (1983).
Cole, A. E. & Shinnick-Gallagher, P. Brain Res. 187, 226–230 (1980).
Ginsborg, B. L., House, C. R. & Silinsky, E. M. J. Physiol., Lond. 236, 723–731 (1974).
Meech, R. W. & Standen, N. B. J. Physiol., Lond. 249, 211–239 (1975).
Barrett, E. F. & Barrett, J. N. J. Physiol., Lond. 255, 737–774 (1976).
Nicoll, R. A. & Alger, B. Science 210, 1122–1124 (1980).
Hotson, J. R. & Prince, D. A. J. Neurophysiol. 43, 409–419 (1980).
Schwartzkroin, P. A. & Stafstrom, C. E. Science 210, 1125–1126 (1980).
Shinnick-Gallaher, P., Yoshimura, M. & Gallagher, J. P. Neurosci. Abstr. 8, 555 (1982).
Cole, A. E. & Nicoll, R. A. Science (in the press).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cole, A., Shinnick-Gallagher, P. Muscarinic inhibitory transmission in mammalian sympathetic ganglia mediated by increased potassium conductance. Nature 307, 270–271 (1984). https://doi.org/10.1038/307270a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/307270a0
This article is cited by
-
Adrenaline inhibits muscarinic transmission in bullfrog sympathetic ganglia
Pflügers Archiv - European Journal of Physiology (1989)
-
Acetylcholine hyperpolarizes central neurones by acting on an M2 muscarinic receptor
Nature (1986)
-
On the mechanism of activation of muscarinic K+ channels by adenosine in isolated atrial cells: involvement of GTP-binding proteins
Pfl�gers Archiv European Journal of Physiology (1986)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.