Abstract
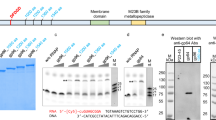
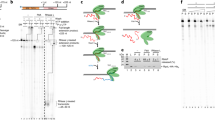
Bacteriophage T4 late transcription is unusual, among prokaryotes, in its complexity. Late transcription requires the host RNA polymerase, the products of T4 genes 33, 45 and 55, and other small polypeptides, the genes of which have not been identified1. In addition the DNA template must be ‘competent’ for late transcription. First the DNA must contain the substituted base 5-hydroxymethyl cytosine in place of cytosine (this requirement is eliminated by a mutation in the T4 alc gene)1,2. Second, the DNA must be replicating, although late transcription can be uncoupled from DNA replication by mutations in the T4 genes coding for DNA ligase (gene 30) and DNA exonuclease (gene 46)1,3. We report here the location of the initiation sites of the messenger RNAs (mRNAs) synthesized in vivo from four late genes (genes 21, 22, 23 and 36) by S1 nuclease mapping and we have determined the DNA sequences at these sites. We have found strong homology to the sequence TATAAATAC-TATT immediately upstream from the 5′ ends of the late messages and we suggest that this sequence is specifically recognized by the complex responsible for late transcription. Also, we have examined gene 23 mRNA synthesized in the absence of DNA replication using the 30− 46− mutant described above and find that it is identical to the true late transcript synthesized in normal infections.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Rabussay, D. & Geiduschek, E. P. in Comprehensive Virology Vol. 8 (eds Fraenkel-Conrat, H. & Wagner, R. R.) 1–196 (Plenum, New York, 1977).
Snyder, L., Gold, L. & Kutter, E. M. Proc. natn. Acad. Sci. USA 69, 603–607 (1972).
Wu, R., Geiduschek, E. P. & Cascino, A. J. molec. Biol. 96, 539–562 (1975).
Young, E. T., Menard, R. C. & Harada, J. J. Virol. 40, 790–799 (1981).
Kassavetis, G. A. & Geiduschek, E. P. EMBO J. 1, 107–114 (1982).
Parker, M., Christensen, A. C., Young, E. T. & Doermann, A. H. (in preparation).
King, J. & Laemmli, U.K. J. molec. Biol. 62, 465–477 (1971).
Oliver, D. B. & Crowther, R. A. J. molec. Biol. 153, 545–568 (1981).
Berk, A. J. & Sharp, P. A. Cell 12, 721–732 (1977).
Nasmyth, K. A., Tatchell, K., Hall, B. D., Astell, C. & Smith, M. Cold Spring Harb. Symp. quant. Biol. 45, 961–981 (1980).
Maxam, A. M. & Gilbert, W. Meth. Enzym. 65, 499–560 (1980).
Sollner-Webb, B. & Reeder, R. H. Cell 18, 485–499 (1979).
Emrich-Owen, J., Schultz, D. W., Taylor, A. & Smith, G. R. J. molec. Biol. (submitted).
Lewis, M. K. & Burgess, R. R. J. biol. Chem. 255, 4928–4936 (1980).
Pribnow, D. in Biological Regulation and Development Vol. 1 (ed. Goldberger, R. F.) 219–278 (Plenum, New York, 1979).
Rosenburg, M. & Court, D. A. Rev. Genet. 13, 319–353 (1979).
Siebenlist, U., Simpson, R. B. & Gilbert, W. Cell 20, 269–281 (1981).
Rosa, M. D. Cell 16, 815–825 (1979).
Lee, G. & Pero, J. J. molec. Biol. 152, 247–265 (1981).
Grosveld, G. C., deBoer, E., Shewmaker, C. K. & Flavell, R. A. Nature 295, 120–126 (1982).
Mattson, T., Van Houwe, G., Bolle, A., Selzer, G. & Epstein, R. Molec. gen. Genet. 154, 319–326 (1977).
Young, E. T. et al. J. molec. Biol. 183, 423–445 (1980).
Völcker, T. A. & Showe, M. K. Molec. gen. Genet. 177, 447–452 (1980).
Showe, M. K., Isobe, E. & Onorato, L. J. molec. Biol. 107, 35–54 (1976).
Hagen, F. & Young, E. T. J. Virol. 26, 793–804 (1978).
Krisch, H. et al. Proc. natn. Acad. Sci. USA (in the press).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Christensen, A., Young, E. T4 late transcripts are initiated near a conserved DNA sequence. Nature 299, 369–371 (1982). https://doi.org/10.1038/299369a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/299369a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.