Abstract
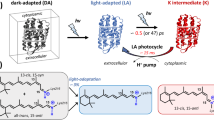
LIGHT absorption by the purple membrane protein (bacterio-rhodopsin) of Halobacterium halobium leads to pumping of protons across the cell membrane1; the resulting proton gradient can in turn be used to make ATP2,3. As in photosynthesis, the energy for this proton pumping must be ultimately derived from the energy of the absorbed photon which is converted into chemical free energy in the formation of the primary photochemical product, bathobacteriorhodopsin or K (refs 4 and 5) (see Fig. 1). On warming, K decays to the photocycle intermediate L and then to M4, which, after warming to higher temperatures, returns to purple membrane protein (PM). The chromophore of the native pigment is all-trans retinal attached to the protein by way of a protonated Schiff base6,7. The question arises : What is the action of light on the chromophore which results in K formation? In earlier papers5,16, we have provided a partial answer to the nature of K by marshalling strong evidence that, as in visual pigments8, the primary effect of light is to isomerise the chromophore. Pettei et al.9 have recently shown using a chromophore extraction method that by the time of M formation, at least part and perhaps all of the chromophores have been isomerised to the 13-cis conformation. Resonance Raman studies of the chromophore of the pigment and its M intermediate also suggest that the chromophore is isomerised from the all-trans to the 13-cis conformation by the time of M formation7. We show here that when M or L is irradiated at −196 °C, their primary photoproducts—M′ or L′—can reform the original pigment when warmed to −90 °C. This property of these new photoproducts can most readily be explained if light has photoisomerised the chromophore of the intermediates back to the conformation originally present in the pigment. Thus, by the L and M intermediates in the photocycle, light has altered the geometry of the chromophore from the all-trans to another geometrical conformation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Oesterhelt, D. & Stoeckenius, W. Proc. natn. Acad. Sci. U.S.A. 70, 2853–2857 (1973).
Danon, A. & Stoeckenius, W. Proc. natn. Acad. Sci. U.S.A. 71, 1234–1238 (1974).
Racker, E. & Stoeckenius, W. J. biol. Chem. 219, 662–663 (1974).
Lozier, R. H., Bogomolni, R. A. & Stoeckenius, W. Biophys. J. 15, 955–960 (1975).
Rosenfeld, T., Honig, B., Ottolenghi, M., Hurley, J. & Ebrey, T. Pure appl. Chem. 49, 341–351 (1977).
Lewis, A., Spoonhower, J., Bogomolni, R. A., Lozier, R. H. & Stoeckenius, W. Proc. natn. Acad. Sci. U.S.A. 71, 4462–4466 (1974).
Aton, B., Doukas, A. G., Callendar, R. H., Becher, B. & Ebrey, T. G. Biochemistry 16, 2995–2999 (1977).
Kropf, A. & Hubbard, R. Ann. N.Y. Acad. Sci. 74, 266–280 (1958).
Pettei, M. J., Yudd, A. P., Nakanishi, K., Henselman, R. & Stoeckenius, W. Biochemistry 16, 1955–1959 (1977).
Fransen, M. R. et al. Nature 260, 726–727 (1976); Thomson, A. Nature 254, 178–179 (1975); Peters, K., Applebury, M. L. & Rentzepis, P. M. Proc. natn. Acad. Sci. U.S.A. 74, 3119–3123 (1977).
Becher, B. & Ebrey, T. Biophys. J. 17, 185–191 (1977).
Kalisky, O., Vri, L. & Ottolenghi, M. Biophys. J. (in the press).
Hess, B. & Kuschmitz, D. FEBS Lett. 74, 20–23 (1977).
Hubbard, R., Brown, P.K. & Kropf, A. Nature 183, 442–450 (1959).
Yoshizawa, T. & Wald, G. Nature 197, 1279–1286 (1963).
Becher, B., Tokunaya, F. & Ebrey, T. Biochemistry (in the press).
Hurley, J. B., Ebrey, T., Honig, B. & Ottolenghi, M. Nature 270, 540–543 (1977).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
HURLEY, J., BECHER, B. & EBREY, T. More evidence that light isomerises the chromophore of purple membrane protein. Nature 272, 87–88 (1978). https://doi.org/10.1038/272087a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/272087a0
This article is cited by
-
Light isomerizes the chromophore of bacteriorhodopsin
Nature (1980)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.