Abstract
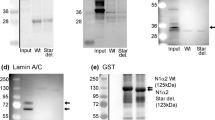
NEMALINE myopathy, a congenital neuromuscular disease, is one of several muscle disorders in which apparently abnormal Z lines, or Z line-type structures emanating from Z lines, has been described1–6. There has been considerable speculation concerning the chemical composition and structural arrangement of proteins in nemaline rods2,4,7–12, but these features have remained unclear: part of this uncertainty is related to lack of understanding of the intact Z line. The only Z line constituent for which there is substantial evidence is α-actinin13–16. We have described the preparation and properties of a Ca2+-activated neutral protease (termed CAF) from muscle that is highly specific in its activity towards myofibrillar proteins and structure17,18. Addition of CAF to isolated myofibrils or to teased muscle fibrils releases undegraded α-actinin from the Z line, with no noticeable effect on myosin, actin, or the remaining myofibrillar structure. We report here the use of CAF as a dissection tool to strip away the dense, amorphous component of the nemaline rods, exposing an underlying set of longitudinal filaments running parallel to the long axis of the original rod. Decoration of these filaments with heavy meromyosin19 shows that the longitudinal filaments of nemaline rods are composed of actin.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Shy, G. M., Engel, W. K., Somers, J. E. & Wanko, T. Brain 86, 793–810 (1963).
Price, H. M., Gordon, G. B., Pearson, C. M., Munsat, T. L. & Blumberg, J. M. Proc. natn. Acad. Sci. U.S.A. 54, 1398–1406 (1965).
Cornog, J. L. & Gonatas, N. K. J. ultrastruct. Res. 20, 433–450 (1967).
Fawcett, D. W. J. Cell Biol. 36, 266–270 (1968).
MacDonald, R. D. & Engel, A. G. Acta neuropath. 14, 99–107 (1969).
Fisher, E. R., Wissinger, H. A., Gerneth, J. A. & Danowski, T. S. Arch. Path. 94, 456–460 (1972).
Engel, A. G. & Gomez, M. R. J. Neuropath. exp. Neurol. 26, 601–619 (1967).
MacDonald, R. D. & Engel, A. G. J. Cell Biol. 48, 431–437 (1971).
Sugita, H., Masaki, T., Ebashi, S. & Pearson, C. M. Proc. Japan Acad. 50, 237–240 (1974).
Yamaguchi, M., Cassens, R. G. & Dahl, D. S. Cytobiologie 11, 335–345 (1975).
Sreter, F. A., Astrom, K.-E., Romanul, F. C. A., Young, R. R. & Jones, H. R. J. Neurol. Sci. 27, 99–116 (1976).
Stromer, M. H., Tabatabai, L. B., Robson, R. M., Goll, D. E. & Zeece, M. G. Expl. Neurol. 50, 402–421 (1976).
Masaki, T., Endo, M. & Ebashi, S. J. Biochem., Tokyo 62, 630–632 (1967).
Robson, R. M., Goll, D. E., Arakawa, N. & Stromer, M. H. Biochim. biophys. Acta 200, 296–318 (1970).
Schollmeyer, J. V. et al. J. Cell Biol. 63, 303a, Abstr. (1974).
Suzuki, A. et al. J. biol. Chem. 251, 6860–6870 (1976).
Dayton, W. R., Goll, D. E., Zeece, M. G., Robson, R. M. & Reville, W. J. Biochemistry 15, 2150–2158 (1976).
Dayton, W. R., Reville, W. J., Goll, D. E. & Stromer, M. H. Biochemistry 15, 2159–2167 (1976).
Huxley, H. E. J. molec. Biol. 7, 281–308 (1963).
Dahl, D. S. & Klutzow, E. W. J. Neurol. Sci. 23, 371–385 (1974).
Engel, A. G. Mayo Clin. Proc. 41, 713–741 (1966).
Schollmeyer, J. E., Goll, D. E., Robson, R. M. & Stromer, M. H. J. Cell Biol. 59, 306a, Abstr. (1973).
Sugita, H., Masaki, T., Ebashi, S. & Pearson, C. M. in Basic Research in Myology, Proc. II Int. Congr. Muscle Dis. (ed. Kakulas, B. A.) 298–302 (American Elsevier, New York, 1973).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
YAMAGUCHI, M., ROBSON, R., STROMER, M. et al. Actin filaments form the backbone of nemaline myopathy rods. Nature 271, 265–267 (1978). https://doi.org/10.1038/271265a0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/271265a0
This article is cited by
-
Skeletal muscle α-actin diseases (actinopathies): pathology and mechanisms
Acta Neuropathologica (2013)
-
Thin filament proteins mutations associated with skeletal myopathies: Defective regulation of muscle contraction
Journal of Molecular Medicine (2008)
-
Hereditary myopathy of the diaphragmatic muscles in Holstein-Friesian cattle
Acta Neuropathologica (1995)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.