Abstract
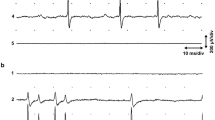
SIGNAL transduction between nerve and muscle is mediated by a chemical process at the neuromuscular junction. In the nerve ending electrical activity causes the release of acetylcholine (ACh) into the synaptic cleft separating the nerve and muscle cells. ACh can bind to specific chemical receptors in the postjunctional muscle membrane; these receptors control a gating mechanism that can increase membrane conductance locally. Normal nervous activity triggers an action potential and contraction in twitch muscle fibres. At constant membrane potential the membrane conductance change induces a current that is carried largely by Na+ and K+ in frog1, moving through ACh-gated membrane channels. There has been a controversy regarding the nature of the chemically gated endplate channels; are there kinetically-independent channels for Na+ and K+ , or are these ions transported by the same channel? Maeno2 initially proposed separate endplate channels, arguing that the two component decay of endplate potentials in procaine-treated cells resulted from a selective effect on Na+ channel kinetics. Kordas3,4 and Magleby and Stevens5 argued that Maeno's two-channel hypothesis could not account for the unique reversal potential observed or the monotonic voltage dependence of endplate current (EPC) decay. The analysis below of iontophoretically-induced EPC fluctuations in procaine-treated and untreated preparations indicates that the ions which constitute the EPC are not carried by kinetically distinguishable endplate channels.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Takeuchi, A. & Takeuchi, N. J. Physiol., Lond. 154, 52–67 (1960).
Maeno, T. J. Physiol., Lond. 183, 592–606 (1966).
Kordas, M. J. Physiol., Lond. 204, 493–502 (1969).
Kordas, M. J. Physiol., Lond. 209, 689–699 (1970).
Magleby, K. L. & Stevens, C. F. J. Physiol., Lond. 223, 173–197 (1972).
Colquhoun, D., Dionne, V. E., Steinbach, J. H. & Stevens, C. F. Nature 253, 204–206 (1975).
Katz, B. & Miledi, R. Nature 226, 962–963 (1970).
Dionne, V. E. & Stevens, C. F. J. Physiol., Lond. 251, 245–270 (1975).
Ruff, R. L. J. Physiol., Lond. (in press).
Anderson, C. R. & Stevens, C. F. J. Physiol., Lond. 235, 655–691 (1973).
Mallart, A., Dreyer, F. & Peper, K. Pflugers Arch. ges. Physiol. 362, 43–47 (1976).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
DIONNE, V., RUFF, R. Endplate current fluctuations reveal only one channel type at frog neuromuscular junction. Nature 266, 263–265 (1977). https://doi.org/10.1038/266263a0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/266263a0
This article is cited by
-
Properties of a Ca2+ activated K+ conductance in /Helix neurones investigated by intracellular Ca2+ ionophoresis
Pfl�gers Archiv European Journal of Physiology (1982)
-
Desensitisation does not selectively alter sodium channels
Nature (1977)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.