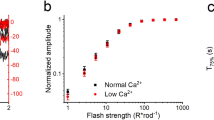
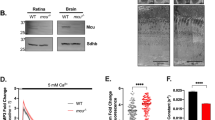
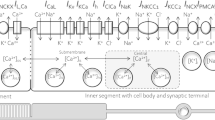
Abstract
THE bleaching of as little as one molecule of rhodopsin can trigger hyperpolarisation of the vertebrate visual receptor cell1. A light modulated Ca2+ flux may function in the receptor cell outer segments (OS) as an intracellular messenger amplifying and transducing the photochemical signal from its disk location to the external plasma membrane2,3. In support of this hypothesis, Ca2+ has been shown to be concentrated in dark adapted disks4 and to be released on bleaching5–7. This suggests the presence of a Ca2+-dependent ATPase, an enzyme often associated with cellular Ca2+ pumps. The report that either the bleaching of a few per cent of the rhodopsin molecules within a frog rod suspension or the intra-OS elevation of Ca2+ to 10−7 M or greater, by means of ionophore A23187, can induce a rapid and very large decrease in ATP content8 provides indirect evidence for the presence of a Ca2+-activated ATPase. Interpretation of this finding is difficult, however, due to other known light-activated, ATP-dependent OS functions9. We present here direct evidence that purified bovine OS exhibit significant Ca2+-dependent ATPase activity, which seems to be independent of contributions from subcellular contamination.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hecht, S., Shlaer, S., and Pirenne, M. H., J. gen. Physiol., 25, 819–840 (1942).
Hagins, W. A., and Yoshikami, S., Expl Eye Res., 18, 299–305 (1974).
Zuckerman, R., J. Physiol., 235, 333–354 (1973).
Bownds, D., Gordon-Walker, A., Gaide-Huguenin, A.D., and Robinson, W. E., J. gen. Physiol., 58, 225–237 (1971).
Hendriks, T., Daemen, F. J. M., and Bonting, S. L., Biochim. biophys. Acta, 345, 468–473 (1974).
Poo, M. M., and Cone, R. A., Expl Eye Res., 17, 503–510 (1973).
Mason, W. T., Fager, R. S., and Abrahamson, E. W., Nature, 247, 562–563 (1974).
Carretta, A., and Cavaggioni, A., J. Physiol. 257, 687–697 (1976).
Weller, M., Goridis, C., Virmaux, N., and Mandel, P., Expl Eye Res., 21, 405–407 (1975).
Erhardt, F., Ostroy, S. E. and Abrahamson, E. W., Biochim. biophys. Acta, 112, 256–264 (1966).
Raubach, R. A., Franklin, L. K., and Dratz, E. A., Vision Res. 14, 335–337 (1973).
McConnell, D. G., J. Cell Biol., 27, 459–473 (1965).
Rossi, C. S., and Lehninger, A. L., Biochem. Z., 338, 698–713 (1963).
Brierley, G. P., Murer E., and Green, D. E., Science, 140, 60–62 (1963).
Huijing, F., and Slater, E. C., J. Biochem., 49, 493–501 (1961).
Butcher, R. W., and Sutherland, E. W., J. biol. Chem., 237, 1244–1250 (1962).
Baginski, E. S., Foa, P. P., and Zak, B., Clin. chim. Acta, 15, 155–158 (1967).
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J., J. biol. Chem. 193, 265–275 (1951).
Welcher, E. J., The Analytical Uses of Ethylenediaminetetraacetic Acid (Van Nostrand, Princeton, 1958).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
SACK, R., HARRIS, C. Ca2+-dependent ATPase activity of bovine receptor cell outer segment. Nature 265, 465–466 (1977). https://doi.org/10.1038/265465a0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/265465a0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.