Abstract
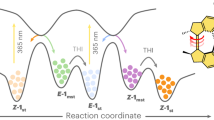
IN the photolysis of the visual pigment rhodopsin the intermediate first observed is bathorhodopsin1,2 (formerly called prelumirhodopsin). It is generally held that isomerisation of 11-cis to all-trans retinal occurs during this initial step. On theoretical grounds, however, an hexaene–amine structure (Fig. 1) has been proposed as an alternative for the chromophore of bathorodopsin (ref. 3 and unpublished results of K. Van der Meer, J. J. C. Mulder and J.L.). This chromophore has an exomethylene double bond between the atoms C5 and C18 and the nitrogen of its ene–amine moiety derives from the ɛ-amino group of a lysine residue of opsin. It is a retrotautomer of the chromophoric group in native rhodopsin which is a protonated Schiff base of 11-cis retinal. We now present two lines of evidence which support the structure depicted in Fig. 1: first, the interpretation of a recently published laser resonance Raman spectrum of bathorhodopsin4, and second, experiments which establish hydrogen (deuterium) exchange during photolysis of rhodopsin.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Yoshizawa, T., and Kito, Y., Nature, 182, 1604–1605 (1958).
Busch, G. E., Applebury, M. L., Lamola, A. A., and Rentzepis, P. M., Proc. natn. Acad. Sci. U.S.A., 69, 2802–2806 (1972).
Kropf, A., in Proc. Int. School of Physics Enrico Fermi, Course 43, 28–43 (Academic, New York, 1963).
Oseroff, A. R., and Callender, R. H., Biochemistry, 13, 4243–4248 (1974).
Sheppard, N., and Simpson, D. M., Q. Rev., Lond., 6, 1–33 (1952).
Patnak, C. M., and Fletcher, W. H., J. molec. Spec., 31, 32–53 (1969).
Nevgi, G. V., and Jatkar, S. K., J. Indian Inst. Sci., 17 A, 189–196 (1934).
Dupont, G., Daure, P., and Allard, J., Bull. Soc. Chim. Fr., 49, 1401–1409 (1931).
De Grip, W. J., Daemen, F. J. M., and Bonting, S. L., Vision Res., 12, 1697–1707 (1972).
Lin, R. L., Waller, G. R., Mitchell, E. D., Yang, K. S., and Nelson, E. C., Analyt. Biochem., 35, 435–441 (1970).
Beynon, J. H., and Williams, A. E., Mass and Abundance Tables for use in Mass Spectrometry (Elsevier, Amsterdam, 1963).
Wald, G., Nature, 219, 800–807 (1968).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
FRANSEN, M., LUYTEN, W., VAN THUIJL, J. et al. Structure of the chromophoric group in bathorhodopsin. Nature 260, 726–727 (1976). https://doi.org/10.1038/260726a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/260726a0
This article is cited by
-
Evidence for a vibrational phase-dependent isotope effect on the photochemistry of vision
Nature Chemistry (2018)
-
Quantum-mechanical kinetic study of the primary reaction of the photochemical cycle of Halobacterium halobium
Biophysics of Structure and Mechanism (1981)
-
More evidence that light isomerises the chromophore of purple membrane protein
Nature (1978)
-
Rhodopsin and its thermal intermediates: Fast structural fluctuations in their protein component
Journal of Biological Physics (1978)
-
Temperature and wavelength effects on the photochemistry of rhodopsin, isorhodopsin, bacteriorhodopsin and their photoproducts
Nature (1977)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.