Abstract
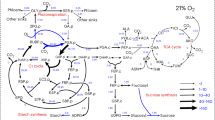
RIBULOSE 1,5-diphosphate carboxylase/oxygenase (fraction 1 protein), the most abundant protein in nature, is a key photosynthetic enzyme catalysing the addition of CO2 to ribulose 1,5-diphosphate (RuDP) to form two molecules of phosphoglyceric acid (PGA). The enzyme also has a competitive oxygenase function catalysing the splitting of RuDP by O2 to form one molecule of PGA and one molecule of phosphoglycolate1–3. The latter function of this enzyme has been suggested to be the primary source of phosphogly-colate, the principal substrate for photorespiration5.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Laing, W. A., Ogren, W. L., and Hageman, R. H., Pl. Physiol. Lancaster, 54, 678–685 (1974).
Badger, M. R., and Andrews, T. J., Biochem. biophys. Res. Commun., 60, 204–210 (1974).
Bahr, J. T., and Jensen, R. G., Archs Biochem. Biophys., 164, 408–413 (1974).
Tolbert, N. E., Curr. Top. cell. Regul., 7, 21–49 (1973).
Zelitch, I., Science, 188, 626–633 (1975).
Baker, T. S., Eisenberg, D., Eiserling, F. A., and Weissman, J., J. molec. Biol., 91, 391–399 (1975).
Sakano, K., Kung, S. D., and Wildman, S. G., Molec. gen. Genet., 130, 91–97 (1974).
Blair, G. E., and Ellis, R. J., Biochim. biophys. Acta, 319, 223–234 (1973).
Nishimura, M., and Akazawa, T. M., Biochemistry, 76, 169–176 (1974).
Gray, J. C., and Kekwick, G. O., Eur. J. Biochem., 44, 481–489 (1974).
Sakano, K., Partridge, J. E., and Shannon, L. M., Biochim. biophys. Acta, 329, 339–341 (1973).
Burk, L. G., and Menser, H. A., Tobacco Sci., 8, 101–106 (1974).
Zelitch, I., and Day, P. R., Pl. Physiol. Lancaster, 43, 1838–1844 (1968).
Zelitch, I., Proc. natn. Acad. Sci. U.S.A., 70, 579–584 (1973).
Kung, S. D., Sakano, K., and Wildman, S. G., Biochim. biophys. Acta, 365, 138–147 (1974).
Singh, S., and Wildman, S. G., Molec. gen. Genet., 124, 187–196 (1973).
Marsho, T. V., and Kung, S. D., Archs Biochem. Biophys. (in the press).
Lorimer, G. H., and Andrews, T. J., Nature, 243, 359–360 (1973).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
KUNG, S., MARSHO, T. Regulation of RuDP carboxylase/oxygenase activity and its relationship to plant photorespiration. Nature 259, 325–326 (1976). https://doi.org/10.1038/259325a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/259325a0
This article is cited by
-
Discoveries in Rubisco (Ribulose 1,5-bisphosphate carboxylase/oxygenase): a historical perspective
Photosynthesis Research (2007)
-
Characterization of the thylakoid membranes of the tobacco aurea mutant Su/su and of three green revertant plants
Planta (1981)
-
The effects of nuclear mutation on chloroplast development
Theoretical and Applied Genetics (1981)
-
Non-mendelian mutation affecting ribulose-1, 5-bisphosphate carboxylase structure and activity
Nature (1980)
-
The effect of divalent metal ions on the activity of Mg++ depleted ribulose-1,5-bisphosphate oxygenase
Planta (1979)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.