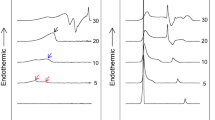
Abstract
FROM many recent studies on the phospholipid–sterol interaction, it has become increasingly clear that the 3 α-and 3 β-hydroxy isomers of a range of sterols have quite different effects on the molecular mobility and functional property of lipid bilayer membranes1,2. For example, the addition of cholesterol or Δ5-cholesten-3 β-ol to liposomes decreases their permeability, whereas epicholesterol, a 3 α-isomer of cholesterol, exerts no apparent effect. It has been proposed that the 3 β-hydroxyl group engages in hydrogen bonding with the carbonyl oxygen of the fatty acyl groups in phospholipids in the bilayer3. Evidence supporting such hydrogen bond formation is strong4. The problem is, then, why does the 3 β-OH group of cholesterol, but not the 3 α-OH group of epicholesterol form such bonds?
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Silbert, D. F., A. Rev. Biochem., 44, 315–339 (1975).
Lawrence, D. K., and Gill, E. W., Molec. Pharmac., 11, 280–286 (1975).
Brockerhoff, H., Lipids, 9, 645–650 (1974).
Yeagle, P. L., Hutton, W. C., Huang, C., and Martin, R. B., Proc. natn. Acad. Sci. U.S. A., 72, 3477–3481 (1975).
Pauling, P., in Structural Chemistry and Molecular Biology, (edit. by Rich, A., and Davidson, N.) 553–565 (W. H. Freeman, San Francisco, 1968).
Dunitz, J. D., and Strickler, P., ibid., 593–602.
Mathieson, A. McL., Tetrahedron Lett., 46, 4137–4138 (1965).
Levine, Y. K., Birdsall, N. J. M., Lee, A. G., and Metcalfe, J. C., Biochemistry, 11, 1416–1421 (1972).
Breitmaier, E., Spohn, K.-H., Berger, S., Angew Chem. int. Edit., 14, 144–159 (1975).
Tardieu, A., Luzzati, V., and Reman, F. C., J. molec. Biol., 15, 711–733 (1973).
Kroon, J., and Kanters, J. A., Nature, 248, 667–668 (1974).
Hasengawa, M., and Noda, H., Nature, 254, 212 (1975).
Levine, Y. K., Progr. Biophys. molec. Biol., 24, 1–74 (1972).
Adam, N. K., Danielli, J. F., Haslewood, G. A. D., and Marrian, G. F., Biochem. J., 26, 1233–1239 (1932).
Hamilton, J. A., Talkowski, C., Childers, R. F., Williams, E., Allerhand, A., and Cordes, E. H., J. biol. Chem., 249, 4872–4878 (1974).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
HUANG, CH. Roles of carbonyl oxygens at the bilayer interface in phospholipid–sterol interaction. Nature 259, 242–244 (1976). https://doi.org/10.1038/259242a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/259242a0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.