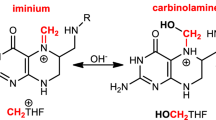
Abstract
IT has been suggested that N-methylation of biogenic amines is involved in the development of certain schizophrenic disorders1,2. Interest in this transmethylation hypothesis has been boosted by the reported identification of a mammalian N-methyltransferase (“dopamine methyltransferase”3) which uses as methyl donor N5-methyltetrahydrofolate (MTHF) rather than S-adenosyl methionine3–5. Further studies have demonstrated that the products of the incubation of catecholamines or indoleamines with MTHF and the transferase were not N-methylated amines but tetrahydroisoquinoline or tetrahydro-β-carboline alkaloids, respectively6–10. These compounds can be formed readily by the condensation of formaldehyde with the amines and it has therefore been postulated that the enzyme originally identified as a methyltransferase may in fact act to release formaldehyde from MTHF6–10. We provide evidence that the enzyme methylenetetrahydrofolate reductase (EC 1.1.1.68) is responsible for the reported production of formaldehyde from MTHF.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Matthysse, S., Smith, E. L., Puck, T. T., and Edelman, G. M., Neurosci. Res. Progr. Bull., 10, 446–445 (1972).
Snyder, S. H., and Banerjee, S. P., in Frontiers in Catecholamine Research (edit. by Usdin, E., and Snyder, S. H.), 1133–1138 (Pergamon, Oxford, 1973).
Laduron, P., Nature new Biol., 238, 212–213 (1972).
Laduron, P. M., Gommeren, W. R., and Leysen, J. E., Biochem. Pharmac., 23, 1599–1608 (1974).
Banerjee, S. P., and Snyder, S. H., Science, 182, 74–75 (1973).
Leysen, J., and Laduron, P., FEBS Lett., 47, 299–303 (1974).
Meller, E., Rosengarten, H., Friedhoff, A. J., Stebbins, R. D., and Silber, R., Science, 187, 171–173 (1975).
Lin, R. L., and Narasimhachari, N., Res. Commun. chem. Path. Pharmac., 8, 535–541 (1974).
Mandel, L. R., Rosegay, A., Walker, R. W., Vanden Heuvel, W. J. A., and Rokach, J., Science, 186, 741–743 (1974).
Wyatt, R. J., Erdelyi, E., Doamaral, J. R., Elliott, G. R., Renson, J., and Barchas, J. D., Science, 187, 853–855 (1975).
Kutzbach, C., and Stokstad, E. L. R., Biochim. Biophys. Acta, 250, 459–477 (1971).
Waldmeier, P. C., and Maitre, L., Experientia, 30, 456–458 (1974).
Burton, E. G., and Sallach, H. J., Archs Biochem. Biophys., 166, 483–494 (1975).
Mandell, A. J., Knapp, S., and Hsu, L. L., Life Sci., 14, 1–17 (1974).
Jacobson, K. B., and Kaplan, N. O., J. biol. Chem., 226, 603–613 (1957).
Ungar, F., Tabakoff, B., and Alivisatos, S. G. A., Biochem. Pharmac., 22, 1905–1913 (1973).
Uotila, L., and Koivusalo, M., J. biol. Chem., 249, 7653–7663 (1974).
Davis, V. E., Ann. N. Y. Acad. Sci., 215, 111–115 (1973).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
PEARSON, A., TURNER, A. Folate-dependent 1-carbon transfer to biogenic amines mediated by methylenetetrahydrofolate reductase. Nature 258, 173–174 (1975). https://doi.org/10.1038/258173a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/258173a0
This article is cited by
-
Vitamin B12 deficiency as a cause of severe neurological symptoms in breast fed infant – a case report
Italian Journal of Pediatrics (2020)
-
Amino acid studies in transient acute polymorphic psychosis
Amino Acids (1997)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.