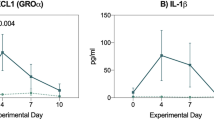
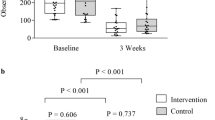
Abstract
Intestinal barrier function was prospectively examined in the course of a clinical trial evaluating the efficacy and safety of lisofylline for reducing cytotoxic therapy-induced intestinal epithelial damage-related infectious morbidity in patients receiving standard remission-induction therapy for acute myeloid leukaemia. The absorption and permeation of oral D-Xylose, lactulose and mannitol were measured weekly from baseline until marrow recovery in adult recipients of idarubicin plus cytarabine for untreated acute myeloid leukaemia. These studies were correlated with non-haematologic chemotherapy-related toxicities reflecting mucosal damage, including nausea, vomiting, stomatitis, diarrhoea, abdominal pain and systemic infection. D-xylose absorption decreased and lactulose:mannitol ratio reflecting intestinal permeability increased from baseline until the second and third week after the beginning of the treatment followed by recovery. These measures correlated with infection rates, nausea, vomiting, diarrhoea and increased blood product utilization. Lisofylline was associated with increased intestinal permeability, nausea, vomiting and infection-related morbidity despite a reduction in the duration of neutropaenia. These surrogates of intestinal barrier function correlated well with clinically important outcomes despite the failure to demonstrate reduced morbidity with lisofylline and represent useful objective outcome measurements for future clinical trials of products for the amelioration of the effects of cytotoxic therapy on the intestinal mucosa.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer-Jensen M et al. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 2004; 100 (Suppl 9): 1995–2025.
Sonis ST . Pathobiology of mucositis. Semin Oncol Nurs 2004; 20: 11–15.
Duncan M, Grant G . Oral and intestinal mucositis – causes and possible treatments. Aliment Pharmacol Ther 2003; 18: 853–874.
Donnelly JP, Blijlevens NM, Verhagen CA . Can anything be done about oral mucositis? Ann Oncol 2003; 14: 505–507.
Rapoport AP, Miller Watelet LF, Linder T, Eberly S, Raubertas RF, Lipp J et al. Analysis of factors that correlate with mucositis in recipients of autologous and allogeneic stem cell transplants. J Clin Oncol 1999; 17: 2446–2453.
Wardley AM, Jayson GC, Swindell R, Morgenstern GR, Chang J, Bloor R et al. Prospective evaluation of oral mucositis in patients receiving myeloablative conditioning regimens and haemopoietic progenitor rescue. Br J Haematol 2000; 110: 292–299.
Blijlevens NM, Donnelly JP, De Pauw BE . Prospective evaluation of gut mucosal barrier injury following various myeloablative regimens for haematopoietic stem cell transplant. Bone Marrow Transplant 2005; 35: 707–711.
Bow EJ, Kilpatrick MG, Scott BA, Clinch JJ, Cheang MS . Acute myeloid leukemia in Manitoba. The consequences of standard ‘7+3’ remission-induction therapy followed by high dose cytarabine postremission consolidation for myelosuppression, infectious morbidity, and outcome. Cancer 1994; 74: 52–60.
Elting LS, Cooksley C, Chambers M, Cantor SB, Manzullo E, Rubenstein EB . The burdens of cancer therapy. Clinical and economic outcomes of chemotherapy-induced mucositis. Cancer 2003; 98: 1531–1539.
Filicko J, Lazarus HM, Flomenberg N . Mucosal injury in patients undergoing haematopoietic progenitor cell transplantation: new approaches to prophylaxis and treatment. Bone Marrow Transplant 2003; 31: 1–10.
Spielberger R, Stiff P, Bensinger W, Gentile T, Weisdorf D, Kewalramani T et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med 2004; 351: 2590–2598.
Cicalese L, Yacoub W, Rogers J, Fung JJ, Rao AS, Starzl TE . Translocation of bacteria from the gastrointestinal tract: protection afforded by lisofylline. Transplant Proc 1999; 31: 575–576.
Wattanasirichaigoon S, Menconi MJ, Delude RL, Fink MP . Lisofylline ameliorates intestinal mucosal barrier dysfunction caused by ischemia and ischemia/reperfusion. Shock 1999; 11: 269–275.
Fegan C, Poynton CH, Whittaker JA . The gut mucosal barrier in bone marrow transplantation. Bone Marrow Transplant 1990; 5: 373–377.
Johansson JE, Ekman T . Gastrointestinal toxicity related to bone marrow transplantation: disruption of the intestinal barrier precedes clinical findings. Bone Marrow Transplant 1997; 19: 921–925.
Johansson JE, Ekman T . Gut mucosa barrier preservation by orally administered IgA–IgG to patients undergoing bone marrow transplantation: a randomised pilot study. Bone Marrow Transplant 1999; 24: 35–39.
Lutgens LC, Blijlevens NM, Deutz NE, Donnelly JP, Lambin P, De Pauw BE . Monitoring myeloablative therapy-induced small bowel toxicity by serum citrulline concentration: a comparison with sugar permeability tests. Cancer 2005; 103: 191–199.
Bow EJ, Loewen R, Cheang MS, Shore TB, Rubinger M, Schacter B . Cytotoxic therapy-induced D-xylose malabsorption and invasive infection during remission-induction therapy for acute myeloid leukemia in adults. J Clin Oncol 1997; 15: 2254–2261.
Bow EJ, Sutherland JA, Kilpatrick MG, Williams GJ, Clinch JJ, Shore TB et al. Therapy of untreated acute myeloid leukemia in the elderly: remission-induction using a non-cytarabine-containing regimen of mitoxantrone plus etoposide. J Clin Oncol 1996; 14: 1345–1352.
Sundstrom GM, Wahlin A, Nordin-Andersson I, Suhr OB . Intestinal permeability in patients with acute myeloid leukemia. Eur J Haematol 1998; 61: 250–254.
Blijlevens NM, van’t LB, Donnelly JP, M’Rabet L, De Pauw BE . Measuring mucosal damage induced by cytotoxic therapy. Support Care Cancer 2004; 12: 227–233.
Blijlevens NM, Lutgens LC, Schattenberg AV, Donnelly JP . Citrulline: a potentially simple quantitative marker of intestinal epithelial damage following myeloablative therapy. Bone Marrow Transplant 2004; 34: 193–196.
National Cancer Institute, National Institutes of Health. Cancer Therapy Evaluation Program, National Cancer Institute Common Toxicity Criteria. Version 2.0. US Department of Health and Human Services: Bethesda, MD, 1999; Report No.: Version 2.0.
Hughes WT, Armstrong D, Bodey GP, Brown AE, Edwards JE, Feld R et al. 1997 Guidelines for the use of antimicrobial agents in neutropenic patients with unexplained fever. Clin Infect Dis 1997; 25: 551–573.
Travis S, Menzies I . Intestinal permeability: functional assessment and significance. Clin Sci (London) 1992; 82: 471–488.
Bjarnason I, MacPherson A, Hollander D . Intestinal permeability: an overview. Gastroenterology 1995; 108: 1566–1581.
Hughes WT, Armstrong D, Bodey GP, Bow EJ, Brown AE, Calandra T et al. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis 2002; 34: 730–751.
Wheatley K, Burnett AK, Goldstone AH, Gray RG, Hann IM, Harrison CJ et al. A simple, robust, validated and highly predictive index for the determination of risk-directed therapy in acute myeloid leukaemia derived from the MRC AML 10 trial. United Kingdom Medical Research Council's Adult and Childhood Leukaemia Working Parties. Br J Haematol 1999; 107: 69–79.
Keefe DM . Gastrointestinal mucositis: a new biological model. Support Care Cancer 2004; 12: 6–9.
Estey EH, Thall PF, Reed P, Kantarjian H, Beran M, Pierce S et al. Treatment of newly diagnosed AML, RAEB-t or RAEB with lisofylline or placebo in addition to chemotherapy. Leukemia 1999; 13: 850–854.
List AF, Maziarz R, Stiff P, Jansen J, Liesveld J, Andrews F et al. A randomized placebo-controlled trial of lisofylline in HLA-identical, sibling-donor, allogeneic bone marrow transplant recipients. The Lisofylline Marrow Transplant Study Group. Bone Marrow Transplant 2000; 25: 283–291.
Sturm A, Dignass AU . Modulation of gastrointestinal wound repair and inflammation by phospholipids. Biochim Biophys Acta 2002; 1582: 282–288.
Meddings JB, Sutherland LR, Byles NI, Wallace JL . Sucrose: a novel permeability marker for gastroduodenal disease. Gastroenterology 1993; 104: 1619–1626.
Smecuol E, Bai JC, Sugai E, Vazquez H, Niveloni S, Pedreira S et al. Acute gastrointestinal permeability responses to different non-steroidal anti-inflammatory drugs. Gut 2001; 49: 650–655.
Meddings JB, Gibbons I . Discrimination of site-specific alterations in gastrointestinal permeability in the rat. Gastroenterology 1998; 114: 83–92.
Anderson AD, Jain PK, Fleming S, Poon P, Mitchell CJ, MacFie J . Evaluation of a triple sugar test of colonic permeability in humans. Acta Physiol Scand 2004; 182: 171–177.
Acknowledgements
We would like to acknowledge and thank the staff of the GD6 Oncology Unit, the Health Sciences Centre, Winnipeg, for their assistance in the care of the patients and in the conduct of this study. Further, we would like to thank Mrs Barbara Freed, Ms Jacquie Thiessen, Mrs Ruth Loewen RN and Mrs Kathy Ramesar RN for their meticulous work in the data collection and management and Mary Cheang M Math for her statistical advice. Funding for this work was derived from a grant-in-aid from Biochem Therapeutics Inc., Montreal, Québec, Canada and Cell Therapeutics Inc., Seattle, WA, USA. The substance of this paper represents original research designed and conducted by EJB. JBM collaborated on the intestinal permeability studies. The results of this study have not been previously presented or published. Interpretation of the study results and discussions regarding publication were made by the investigators without participation of the sponsor. The protocol was approved by the Committee on Use of Human Subjects in Research, the University of Manitoba. All patients provided informed written consent.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bow, E., Meddings, J. Intestinal mucosal dysfunction and infection during remission-induction therapy for acute myeloid leukaemia. Leukemia 20, 2087–2092 (2006). https://doi.org/10.1038/sj.leu.2404440
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2404440
Keywords
This article is cited by
-
Antibiotic Management of Patients with Hematologic Malignancies: From Prophylaxis to Unusual Infections
Current Oncology Reports (2022)
-
The interplay between anticancer challenges and the microbial communities from the gut
European Journal of Clinical Microbiology & Infectious Diseases (2022)
-
Characterization of microbiota in acute leukemia patients following successful remission induction chemotherapy without antimicrobial prophylaxis
International Microbiology (2021)
-
Acute abdomen in the immunocompromised patient: WSES, SIS-E, WSIS, AAST, and GAIS guidelines
World Journal of Emergency Surgery (2021)
-
Elevated plasma phage load as a marker for intestinal permeability in leukemic patients
Medical Microbiology and Immunology (2020)