Abstract
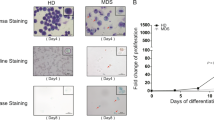
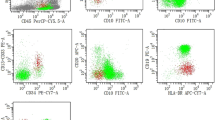
To investigate the relationship between the fetal hemoglobin-containing erythroblasts (F blasts) and apoptosis in myelodysplastic syndromes (MDS), we immunohistochemically assessed F blasts, F cells, and apoptosis in 137 patients with MDS. A marked increase in the number of F blasts in the bone marrow was identified in 116 of 137 patients (84.7%), and the number of F cells was elevated in 54 patients (39.4%). Among the erythroblasts stained by anti-glycophorin C antibody, the mean percentage of F blasts was 14.63 ± 9.17% in MDS, which was significantly higher than that in non-MDS patients with stress erythropoiesis (4.82 ± 3.35%, P < 0.01), although there were no significant differences in the number of F cells between these groups. In particular, 62 of the 137 MDS patients (45.3%) had an apparent increase in F blasts but no elevation of F cells. The apoptotic rate was significantly higher in the patients with a F blast/F cell (Fb/Fc) ratio ⩾5.0 than in those with a Fb/Fc ratio <1.0 (P < 0.01). The results indicate that F cell precursors are incapable of maturing into functioning end-stage F cells, presumably owing to apoptotic cell death. The measurement of F blasts in the bone marrow is needed for the precise evaluation of fetal-type erythropoiesis in MDS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Bard H . The postnatal decline of hemoglobin F synthesis in normal full-term infants J Clin Invest 1975 55: 395–398
Boyer SH, Belding TK, Margolet L, Noyes AN . Fetal hemoglobin restriction to a few erythrocytes (F-cells) in normal human adults Science 1975 188: 361–363
Wood WG, Stomatoyannopoulos G, Lim G, Nute PE . F-cells in the adult: normal values and levels in individuals with hereditary and acquired elevations of Hb F Blood 1975 46: 671–682
Jane SM, Cunningham JM . Understanding fetal globin gene expression: a step towards effective HbF reactivation in haemoglobinopathies Br J Haematol 1998 102: 415–422
Stamatoyannopoulos G, Veith R, Galanello R, Papayannopoulou T . HbF production in stressed erythropoiesis: observations and kinetic models Ann NY Acad Sci 1985 445: 188–197
Bard H, Gagnon C, Peri KG . HbF synthesis during stress erythropoiesis as determined by gamma-RNA/non-alpha-mRNA quantification Pediatr Res 1999 45: 684–686
Reinhardt D, Haase D, Schoch C, Wollenweber S, Hinkelmann E, Heyden WV, Lentini G, Wormann B, Schroter W, Pekrun A . Hemoglobin F in myelodysplastic syndrome Ann Hematol 1998 76: 135–138
Betke K, Marti HR, Schlicht J . Estimation of small percentage of foetal haemoglobin Nature 1959 184: 1877–1878
Bourantas KL, Gergiou J, Seferiadis K . Quantitation of HbF-chain types by HPLC in patients with myelodysplastic syndrome Haematologica 1991 76: 337–338
Bisse E, Wieland H . High-performance liquid chromatographic separation of human haemoglobins: simultaneous quantitation of foetal and glycated haemoglobins J Chromatogr 1988 434: 95–110
Epstein N, Epstein M, Boulet A, Fibach E, Rodgers GP . Monoclonal antibody-based methods for quantitation of hemoglobin: application to evaluating patients with sickle cell anemia treated with hydroxyurea Eur J Haematol 1996 57: 17–24
Campbell TA, Ware RE, Mason M . Detection of hemoglobin variants in erythrocytes by flow cytometry Cytometry 1999 35: 242–248
Chen JC, Bigelow N, Davis BH . Proposed flow cytometric reference method for the determination of erythroid F-cell counts Cytometry 2000 42: 239–246
Choi JW, Kim Y, Fujino M, Ito M . A new anti-hemoglobin F antibody against synthetic peptide for the detection of F-cell precursors (F-blasts) in bone marrow Int J Hematol 2001 74: 277–280
Parker JE, Mufti GJ . Ineffective haematopoiesis and apoptosis in myelodysplastic syndromes Br J Haematol 1998 101: 220–230
Parcharidou A, Raza A, Economopoulos T, Papageorgiou E, Anagnostou D, Papadaki T, Raptis S . Extensive apoptosis of bone marrow cells as evaluated by the in situ end-labelling (ISEL) technique may be the basis for ineffective haematopoiesis in patients with myelodysplastic syndromes Eur J Haematol 1999 62: 19–26
Kudo S, Harigae H, Watanabe N, Takasawa N, Kimura J, Kameoka J, Meguro K, Imaizumi M, Kaku M, Sasaki T . Increased HbF levels in dyserythropoiesis Clin Chim Acta 2000 291: 83–87
Frankfurt OS, Robb JA, Sugarbaker EV, Villa L . Monoclonal antibody to single-stranded DNA is a specific and sensitive cellular marker of apoptosis Exp Cell Res 1996 226: 387–397
Newman DR, Pierre RV, Linman JW . Studies on the diagnostic significance of hemoglobin F levels Mayo Clin Proc 1973 48: 199–202
Passmore SJ, Hann IM, Stiller CA, Ramani P, Swansbury GJ, Gibbons B, Reeves BR, Chessells JM . Pediatric myelodysplasia: a study of 68 children and a new prognostic scoring system Blood 1995 85: 1742–1750
Craig JE, Sampietro M, Oscier DG, Contreras M, Thein SL . Myelodysplastic syndrome with karyotype abnormality is associated with elevated F-cell production Br J Haematol 1996 93: 601–605
Haase D, Fonatsch C, Freund M, Wormann B, Bodenstein H, Bartels H, Stollmann-Gibbels B, Lengfelder E . Cytogenetic findings in 179 patients with myelodysplastic syndromes Ann Hematol 1995 70: 171–187
Shetty V, Hussaini S, Broady-Robinson L, Allampallam K, Mundle S, Borok R, Broderick E, Mazzoran L, Zorat F, Raza A . Intramedullary apoptosis of hematopoietic cells in myelodysplastic syndrome patients can be massive: apoptotic cells recovered from high-density fraction of bone marrow aspirates Blood 2000 96: 1388–1392
Tsoplou P, Kouraklis-Symeonidis A, Thanopoulou E, Zikos P, Orphanos V, Zoumbos NC . Apoptosis in patients with myelodysplastic syndromes: differential involvement of marrow cells in good vs poor prognosis patients and correlation with apoptosis-related genes Leukemia 1999 13: 1554–1563
Yoshida Y, Mufti GJ . Apoptosis and its significance in MDS: controversies revisited Leuk Res 1999 23: 777–785
Merchant SH, Gonchoroff NJ, Hutchison RE . Apoptotic index by Annexin V flow cytometry: adjunct to morphologic and cytogenetic diagnosis of myelodysplastic syndromes Cytometry 2001 46: 28–32
Shimazaki K, Ohshima J, Suzumiya C, Kawasaki C, Kikuchi M . Evaluation of apoptosis as a prognostic factor in myelodysplasitc syndromes Br J Haematol 2000 110: 584–590
Raza A, Gezer S, Mundle S, Gao X, Alvi S, Borok R, Rifkin S, Iftikhar A, Shetty V, Parcharidou A, Loew J, Marcus B, Khan Z, Chaney C, Showel J, Gregory S, Preisler H . Apoptosis in bone marrow biopsy samples involving stromal and hematopoietic cells in 50 patients with myelodysplastic syndromes Blood 1995 86: 268–276
Lepelley P, Campergue L, Grardel N, Preudhomme C, Cosson A, Fenaux P . Is apoptosis a massive process in myelodysplastic syndromes? Br J Haematol 1996 95: 368–371
Weinberg RS, Goldberg JD, Schofield JM, Lenes AL, Styczynski R, Alter BP . Switch from fetal to adult hemoglobin is associated with a change in progenitor cell population J Clin Invest 1983 71: 785–794
Koc A, Oner R, Oner D, Aktas D, Sozen M, Tuncbilek E, Altay C . Myelodysplastic sydrome (MDS) associated with increased hemoglobin F and trisomy 8: presentation of a patient Hematol Cell Ther 1999 41: 187–189
Acknowledgements
This study was supported by a grant from DAIKO Foundation.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Choi, J., Kim, Y., Fujino, M. et al. Significance of fetal hemoglobin-containing erythroblasts (F blasts) and the F blast/F cell ratio in myelodysplastic syndromes. Leukemia 16, 1478–1483 (2002). https://doi.org/10.1038/sj.leu.2402536
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2402536
Keywords
This article is cited by
-
Fetal hemoglobin level predicts lower-risk myelodysplastic syndrome
International Journal of Hematology (2023)
-
The diagnosis from the pathological viewpoint of a blood disease
International Journal of Hematology (2002)