Abstract
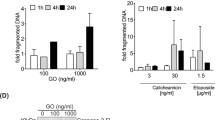
The organosulfur compound ajoene, a constitutent of garlic, has been shown to induce apoptosis in a leukemic cell line as well as in blood cells of a leukemic patient. The mechanisms of action of ajoene, however, are unknown. The present study aims to characterize the molecular events leading to ajoene-triggered apoptosis. We show here that ajoene (20 μM) leads to a time-dependent activation of caspase-3-like activity as well as to the proteolytic processing of procaspase-3 and -8. Activation of caspases was necessary for ajoene-induced apoptosis since the broad-range caspase inhibitor zVAD-fmk completely abrogated ajoene-mediated DNA fragmentation. Although the initiator caspase-8 was activated, the CD95 death receptor was not involved in death signaling since the HL-60 clone used was shown to express a functionally inactive CD95 receptor. Furthermore, ajoene induced the release of cytochrome c, which was not inhibited by zVAD-fmk indicating that cytochrome c release precedes caspase activation. Ajoene also led to a dissipation of the mitochondrial transmembrane potential. Overexpression of Bcl-xL clearly diminished ajoene-induced caspase activation as well as apoptosis. These results indicate that apoptosis in leukemia cells triggered by ajoene is based on the activation of a mitochondria-dependent caspase cascade which includes also the activation of the initiator caspase-8.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Sparnins VL, Barany G, Wattenberg LW . Effects of organosulfur compounds from garlic and onions on benzo[a]pyrene-induced neoplasia and glutathione S-transferase activity in the mouse Carcinogenesis 1988 9: 131–134
Sumiyoshi H, Wargovich MJ . Chemoprevention of 1,2-dimethylhydrazine-induced colon cancer in mice by naturally occurring organosulfur compounds Cancer Res 1990 50: 5084–5087
Zhang Y, Talalay P, Cho C-G, Posner GH . A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure Proc Natl Acad Sci USA 1992 89: 2399–2403
Reddy BS, Rao CV, Rivenson A, Kelloff G . Chemoprevention of colon carcinogenesis by organosulfur compounds Cancer Res 1993 53: 3493–3498
Pinto JT, Qiao C, Xing J, Rivlin RS, Protomastro ML, Weissler ML, Tao Y, Thaler H, Heston WDW . Effects of garlic thioallyl derivatives on growth, glutathione concentration, and polyamine formation of human prostate carcinoma cells in culture Am J Clin Nutr 1997 66: 398–405
Gamet-Payrastre L, Lumeau S, Gasc N, Cassar G, Rollin P, Tulliez J . Selective cytostatic and cytotoxic effects of glucosinolates hydrolysis products on human colon cancer cells in vitro Anticancer Drugs 1998 9: 141–148
Gamet-Payrastre L, Li P, Lumeau S, Cassar G, Dupont M-A, Chevolleau S, Gasc N, Tulliez J, Tercé F . Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells Cancer Res 2000 60: 1426–1433
Sigounas G, Hooker JL, Li W, Anagnostou A, Steiner M . S-allylmercaptocysteine, a stable thioallyl compound, induces apoptosis in erythroleukemia cell lines Nutr Cancer 1997 28: 153–159
Dirsch VM, Gerbes AL, Vollmar AM . Ajoene, a compound of garlic, induces apoptosis in human promyeloleukemic cells, accompanied by generation of reactive oxygen species and activation of nuclear factor κB Mol Pharmacol 1998 53: 402–407
Lee E, Kong G, Lee SJ, Kim ND, Surh YJ . 2-(allylthio)pyrazine suppresses the growth and proliferation of human promyelocytic leukemia (HL-60) cells via induction of apoptosis Anticancer Res 1999 19: 4073–4080
Wong WW-L, MacDonald S, Langler RF, Penn LZ . Novel synthetic organosulfur compounds induce apoptosis of human leukemic cells Anticancer Res 2000 20: 1367–1374
Apitz-Castro R, Cabrera S, Cruz MR, Ledezma E, Jain MK . Effects of garlic extract and of three pure components isolated from it on human platelet aggregation, arachidonate metabolism, release reaction, and platelet ultrastructure Thromb Res 1983 32: 155–169
Kaufmann SH, Earnshaw WC . Induction of apoptosis by cancer chemotherapy Exp Cell Res 2000 256: 42–49
Friesen C, Herr I, Krammer PH, Debatin K-M . Involvement of the CD95 (APO-1/Fas) receptor/ligand system in drug-induced apoptosis in leukemia cells Nat Med 1996 2: 574–577
Müller M, Strand S, Hug H, Heinemann E-M, Walczak H, Hofmann WJ, Stremmel W, Krammer PH, Galle PR . Drug-induced apoptosis in hepatoma cells is mediated by the CD95 (APO-1/Fas) receptor/ligand system and involves activation of wild-type p53 J Clin Invest 1997 99: 403–413
Micheau O, Solary E, Hammann A, Martin F, Dimanche-Boitrel MT . Sensitization of cancer cells treated with cytotoxic drugs to Fas mediated cytotoxicity J Natl Cancer Inst 1997 89: 783–789
Fulda S, Strauss G, Meyer E, Debatin K-M . Functional CD95 ligand and CD95 death-inducing signaling complex in activation-induced cell death and doxorubicin-induced apoptosis in leukemic T cells Blood 2000 95: 301–308
Scaffidi C, Kirchhoff S, Krammer PH, Peter ME . Apoptosis signaling in lymphocytes Curr Opin Immunol 1999 11: 277–285
Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin K-M, Krammer PH, Peter ME . Two CD95 (APO-1/Fas) signaling pathways EMBO J 1998 17: 1675–1687
Villunger A, Egle A, Kos M, Hartmann BL, Geley S, Kofler R, Greil R . Drug-induced apoptosis is associated with enhanced Fas (APO-1/CD95) ligand expression but occurs independently of Fas (APO-1/CD95) signaling in human T-acute lymphatic leukemia cells Cancer Res 1997 57: 3331–3334
Eischen CM, Kottke TJ, Martins LM, Basi GS, Tung JS, Earnshaw WC, Leibson PJ, Kaufmann SH . Comparison of apoptosis in wild-type and Fas-resistant cells: chemotherapy-induced apoptosis is not dependent on Fas/Fas ligand interactions Blood 1997 90: 935–943
Kataoka T, Schröter M, Hahne M, Schneider P, Irmler M, Thome M, Froelich CJ, Tschopp J . FLIP prevents apoptosis induced by death receptors but not by perforin/granzyme B, chemotherapeutic drugs, and gamma irradiation J Immunol 1998 161: 3936–3942
Wesselborg S, Engels IH, Rossmann E, Los M, Schulze-Osthoff K . Anticancer drugs induce caspase-8/FLICE activation and apoptosis in the absence of CD95 receptor/ligand interaction Blood 1999 93: 3053–3063
Kroemer G, Reed JC . Mitochondrial control of cell death Nat Med 2000 6: 513–519
Budihardjo I, Oliver H, Lutter M, Luo X, Wang X . Biochemical pathways of caspase activation during apoptosis Annu Rev Cell Dev Biol 1999 15: 269–290
Ibrado AM, Liu L, Bhalla K . Bcl-xL overexpression inhibits progression of molecular events leading to paclitaxel-induced apoptosis of human acute myeloid leukemia Cancer Res 1997 57: 1109–1115
Peter ME, Dhein J, Ehret A, Hellbardt S, Walczak H, Moldenhauer G, Krammer PH . APO-1 (CD95)-dependent and -independent antigen receptor-induced apoptosis in human T and B cell lines Int Immunol 1995 7: 1873–1877
Walczak H, Bouchon A, Stahl H, Krammer PH . Tumor necrosis factor-related apoptosis-inducing ligand retains its apoptosis-inducing capacity on Bcl-2- or Bcl-xL-overexpressing chemotherapy-resistant tumor cells Cancer Res 2000 60: 3051–3057
Dirsch VM, Stuppner H, Vollmar AM . Helenalin triggers a CD95 death receptor-independent apoptosis that is not affected by overexpression of Bcl-xL or Bcl-2 Cancer Res 2001 61: 5817–5823
Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi CA . A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry J Immunol Methods 1991 139: 271–279
Thornberry NA . Interleukin-1 beta converting enzyme Meth Enzymol 1994 244: 615–631
Bradford MM . A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding Anal Biochem 1976 72: 248–254
Scaffidi C, Medema JP, Krammer PH, Peter ME . FLICE is predominantly expressed as two functionally active isoforms, caspase 8/a and caspase 8/b J Biol Chem 1997 272: 26953–26958
Leist M, Volbracht C, Fava E, Nicotera P . 1-Methyl-4-phenylpyridinium induces autocrine excitotoxicity protease activation, and neuronal apoptosis Mol Pharmacol 1998 54: 789–801
Kidd VJ . Proteolytic activities that mediate apoptosis Annu Rev Physiol 1998 60: 533–573
Medema JP, Scaffidi C, Kischkel FC, Shevchenko A, Mann M, Krammer PH, Peter ME . FLICE is activated by association with the CD95 death-inducing signaling complex (DISC) EMBO J 1997 16: 2794–2804
Clément M-V, Hirpara JL, Chawdhury S-H, Pervaiz S . Chemopreventive agent resveratrol, a natural product derived from grapes, triggers CD95 signaling-dependent apoptosis in human tumor cells Blood 1998 92: 996–1002
Gajate C, Fonteriz RI, Cabaner C, Alvarez-Noves G, Alvarez-Rodriguez Y, Modolell M, Mollinedo F . Intracellular triggering of Fas, independently of FasL, as a new mechanism of antitumor ether lipid-induced apoptosis Int J Cancer 2000 85: 674–682
Ohashi M, Iwase M, Nagumo M . Changes in susceptibility to Fas-mediated apoptosis during differentiation of HL-60 cells J Leukoc Biol 2000 67: 374–380
Loeffler M, Kroemer G . The mitochondrion in cell death control: certainties and incognita Exp Cell Res 2000 256: 19–26
Bratton SB, MacFarlane M, Cain K, Cohen GM . Protein complexes activate distinct caspase cascades in death receptor and stress-induced apoptosis Exp Cell Res 2000 256: 27–33
Cossarizza A, Ceccarelli D, Masini A . Functional heterogeneity of an isolated mitochondrial population revealed by cytofluorometric analysis at the single organelle level Exp Cell Res 1996 222: 84–94
Gross A, McDonnell JM, Korsmeyer SJ . Bcl-2 family members and the mitochondria in apoptosis Genes Dev 1999 13: 1899–1911
Earnshaw WC, Martins LM, Kaufmann SH . Mammalian caspases: structure, activation, substrates, and functions during apoptosis Annu Rev Biochem 1999 68: 383–424
Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, Wang H-G, Reed JC, Nicholson DW, Alnemri ES, Green DR, Martin SJ . Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner J Cell Biol 1999 144: 281–292
Tang D, Lahti JM, Kidd VJ . Caspase-8 activation and Bid cleavage contribute to MCF7 cellular execution in a caspase-3-dependent manner during staurosporine-mediated apoptosis J Biol Chem 2000 275: 9303–9307
Leist M, Jäättelä M . Four deaths and a funeral: from caspases to alternative mechanisms Nature Rev Cell Biol 2001 2: 589–598
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 369). We thank Dr PH Krammer and Dr H Walczak (DKFZ, Heidelberg, Germany) for supplying the anti-caspase-8 antibody and all Jurkat cell lines, Dr KN Bhalla (Moffitt Cancer Center and Research Institute, University of South Florida, Tampa, USA) for providing HL-60/bcl-xL and HL-60/neo cells, T Meindl (GSF, München, Germany) for taking confocal microscopy pictures and T Roos for excellent technical assistance.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dirsch, V., Antlsperger, D., Hentze, H. et al. Ajoene, an experimental anti-leukemic drug: mechanism of cell death. Leukemia 16, 74–83 (2002). https://doi.org/10.1038/sj.leu.2402337
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2402337
Keywords
This article is cited by
-
Synthetic and antitumor comparison of 9-O-alkylated and carbohydrate-modified berberine derivatives
Journal of the Iranian Chemical Society (2020)
-
ROS-mediated activation of JNK/p38 contributes partially to the pro-apoptotic effect of ajoene on cells of lung adenocarcinoma
Tumor Biology (2016)
-
Induction of apoptosis and cell cycle arrest by Negombata magnifica sponge in hepatocellular carcinoma
Medicinal Chemistry Research (2016)
-
Antitumoral effects of cyclin-dependent kinases inhibitors CR8 and MR4 on chronic myeloid leukemia cell lines
Journal of Biomedical Science (2015)
-
Compounds from Allium species with cytotoxic and antimicrobial activity
Phytochemistry Reviews (2014)