Abstract
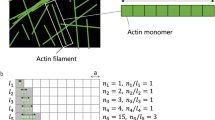
ACTIN filaments appear to be an essential part of the contractile apparatus in all types of muscle. Electron microscope investigations of negatively stained material isolated from a variety of muscles have suggested that these filaments (about 80 Å in diameter) consist of two helically wound strands of globular units about 55 Å in diameter, each unit probably representing one monomer of actin1. This structural picture of actin filaments is in essential agreement with earlier X-ray diffraction studies on whole muscle by Selby and Bear2. Their analysis, however, could not specify the length of the long period (that is, the pitch) of each of the helically wound strands. On the assumption that there is an integral number of monomeric units in each turn of this helix, the long period could be either 2 × 350 Å (thirteen units per turn) or 2 × 410 Å (fifteen units per turn). But there is, in fact, no reason to assume that the number of monomeric units in each turn is integral; the pitch of the helix could also lie anywhere near or between 2 × 350 and 2 × 410 Å.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hanson, J., and Lowy, J., J. Mol. Biol., 6, 46 (1963).
Selby, C. C., and Bear, R. S., J. Biophys. Biochem. Cytol., 2, 71 (1956).
Peterson, R. P. J. Cell. Biol., 18, 213 (1963).
Huxley, H. E., J. Mol. Biol., 7, 281 (1963).
Worthington, C. R., J. Mol. Biol., 1, 398 (1959).
Worthington, C. R., J. Mol. Biol., 3, 618 (1961).
Huxley, H. E., Holmes, K. C., and Brown, W., in Principles of Biomolecular Organisation (edit. by Wolstenholme, G. E. W., and O'Connor, M.), 259 (Churchill, London, 1966).
Elliott, G. F., and Worthington, C. R., J. Ultrastruct. Res., 9, 166 (1963).
Stokes, A. R., in X-ray Diffraction by Polycrystalline Materials (Chapman and Hall, London, 1960).
Elliott, G. F., Lowy, J., and Worthington, C. R., J. Mol. Biol., 6, 295 (1963).
Elliott, G. F., Lowy, J., Millman, B. M., Nature, 206, 1357 (1965).
Lowy, J., Hanson, J., Elliott, G. F., Millman, B. M., and McDonough, M. W., in Principles of Biomolecular Organisation (edit. by Wolstenholme, G. E. W., and O'Connor, M.), 229 (Churchill, London, 1966).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
MILLMAN, B., ELLIOTT, G. & LOWY, J. Axial Period of Actin Filaments: X-ray Diffraction Studies. Nature 213, 356–358 (1967). https://doi.org/10.1038/213356a0
Received:
Issue Date:
DOI: https://doi.org/10.1038/213356a0
This article is cited by
-
Low angle x-ray diffraction and biology
Journal of Biological Physics (1974)
-
Structure and Organization of Actin in a Molluscan Smooth Muscle
Nature (1967)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.