Abstract
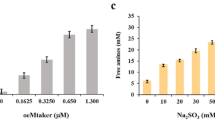
NUMEROUS workers1–3 have fractionated wool keratin after oxidation of disulphide bonds with performic or peracetic acids into three fractions, α, β and γ-keratose (β-keratose is insoluble in water, α-keratose is that fraction precipitating at pH 4 and γ-keratose the remaining fraction). While these fractions vary somewhat in the amounts of terminal amino-acids4, no comments have yet been made on any changes in constitution of the isolated fraction of γ-keratose on re-dissolution in water. As an introduction to some work on the hydrolysis of γ-keratose (prepared by the method of Corfield, Robson and Skinner2), the free amino-nitrogen value was determined by reaction with nitrous acid, the method of Van Slyke5,6, and it was found that reproducible results were not obtained on samples until the solution had been standing for some time. Accordingly the determination of amino-nitrogen of γ-keratose (10 mg/ml.) at various periods of time after dissolution was carried out and plotted as a function of time in acid (0.2 M potassium hydrogen phthalate buffer, pH 4), alkaline (0.2 M boric acid in 0.2 M potassium chloride buffer, pH 10), salt (0.2 M potassium chloride) and simple aqueous solution. The results are shown in Fig. 1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Alexander, P., and Hudson, R. F., Wool: Its Chemistry and Physics (Chapman and Hall, Ltd., London, 1954).
Corfield, M. C., Robson, A., and Skinner, B., Biochem. J., 68, 348 (1958).
O'Donnell, I. J., and Thompson, E. O. P., Austral. J. Biol. Sci., 12, 294 (1959).
Alexander, P., and Smith, L. F., Proc. Intern. Wool Res. Conf., Austral., Vol. B, B–56 (1955).
Milton, R. F., and Waters, W. A., Methods of Quantitative Micro-analysis, 576 (Edward Arnold Ltd., London, 1955).
Van Slyke, D. D., J. Biol. Chem., 83, 425 (1929).
Gornall, A. G., Bardawill, C. J., and David, M. M., J. Biol. Chem., 177, 755 (1949).
Imahori, K., Biochim. Biophys. Acta, 37, 366 (1958).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
ASQUITH, R., WATSON, P. Changes in Amino-nitrogen Content of Solutions of γ-Keratose from Wool Keratin. Nature 208, 786–787 (1965). https://doi.org/10.1038/208786a0
Issue Date:
DOI: https://doi.org/10.1038/208786a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.