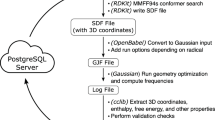
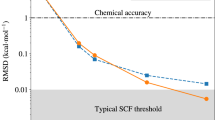
Abstract
ONE of the more successful descriptions of the electronic structure of aromatic hydrocarbons has been given by molecular orbital theory. Originally formulated by Huckel1, it has been developed and modified by many different authors. An account of the molecular orbital theory in its various forms has been given by Daudel, Moser and Lefebvre2. There are two relationships given by molecular orbital theory which are of interest. The first, concerning the ionization potential I of the molecule, is:  where α is the coulomb integral, β the exchange integral and xi is a number characteristic of the molecule.
where α is the coulomb integral, β the exchange integral and xi is a number characteristic of the molecule.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Huckel, E., Z. Phys., 69, 423 (1930); 70, 204 (1931).
Daudel, R., Moser, C., and Lefebvre, R., Quantum Chemistry (Interscience, New York, 1959).
Dewar, M. J. S., J. Chem. Soc., 3532 (1952).
Matsen, F. A., J. Chem. Phys., 24, 602 (1956); Hedges, R. M., and Matsen, F. A., J. Chem. Phys., 28, 950 (1958).
Platt, J. R., J. Chem. Phys., 17, 484 (1949).
Clar, E., Aromatische Kohlenwasserstoffe (Springer-Verlag, Berlin, 1952).
Birks, J. B., and Slifkin, M. A., Nature, 191, 161 (1961).
Brann, A., and Busch, G., Helv. Phys. Acta., 20, 33 (1947).
Heilbronner, E., and Murrel, J. N., J. Chem. Soc., 2611 (1962).
Bond, W. N., Probability and Random Error (Arnold, London, 1935).
Koutechy, J., Pauldus, J., and Zahradnik, R., J. Chem. Phys., 36, 3139 (1962).
Coulson, C. A., Valence (Oxford, 1961).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
SLIFKIN, M. Molecular Orbital Theory and Experimentally Determined Energy-levels. Nature 200, 877–879 (1963). https://doi.org/10.1038/200877b0
Issue Date:
DOI: https://doi.org/10.1038/200877b0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.