Abstract
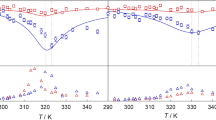
FOR any of a wide variety of pairs of n-alkanes it is known1,2 that at 20° C the heat of mixing is positive. Until about two years ago it was assumed on the basis of the measurements by van der Waals and Hermans1 that as the temperature was increased the heat of mixing of any pair decreased, markedly at first, but remained positive. Then we published2 some measurements of the heat of mixing of n-hexane + n-hexadecane at 20°, 30°, 40°, and 50° C. At 20° C our measurements were in fair agreement with those of van der Waals and Hermans. The temperature-dependence of our measurements was, however, strikingly different from that found by them for other pairs. By extrapolation of our results3 and on theoretical grounds4 we predicted that for n-hexane + n-hexadecane the heat of mixing should change sign at about 64° C and become negative at higher temperatures. That prediction became the subject of a wager (of a good dinner) between members of the ‘Liquids Club’ at the Shell Laboratory in Amsterdam and one of us (M.L.M.) in Reading, and has recently been publicly challenged on theoretical grounds by Holleman and Hijmans5. We have now used a modified version of our calorimeter6 to make a few measurements on equimolar mixtures of n-hexane + n-hexadecane at 40°, 75°, and 100° C. The results of these new measurements are shown plotted against temperature in Fig. 1 together with the results of all the previous measurements made at more than one temperatur.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
van der Waals, J. H., and Hermans, J. J., Rec. Trav. Chim. Pays-Bas, 68, 181 (1949); 69, 949, 971 (1950). van der Waals, J. H., ibid., 70, 101 (1951).
McGlashan, M. L., and Morcom, K. W., Trans. Farad. Soc., 57, 581, 907 (1961).
Ref. 2, 586.
McGlashan, M. L., Morcom, K. W., and Williamson, A. G., Trans. Farad. Soc., 57, 608 (1961).
Holleman, Th., and Hijmans, J., Physica, 28, 604 (1962).
Larkin, J. A., and McGlashan, M. L., J. Chem. Soc., 3425 (1961).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
FRIEND, J., LARKIN, J., MAROUDAS, A. et al. Temperature-dependence of the Heat of Mixing of Two n-Alkanes. Nature 198, 683–684 (1963). https://doi.org/10.1038/198683b0
Issue Date:
DOI: https://doi.org/10.1038/198683b0
This article is cited by
-
Macromolecular conformations in solutions. II. Thermodynamics of interactions
Journal of Statistical Physics (1982)
-
Evidence for coupling of torsional oscillations in alkane mixtures from thermodynamic data, obtained by means of gas chromatography
Journal of Solution Chemistry (1978)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.