Abstract
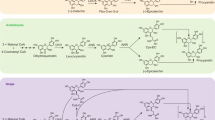
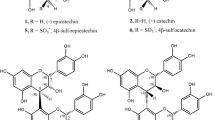
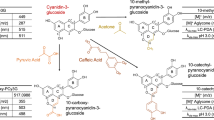
Freudenberg1, Hergert2 and Hathway3 have each suggested several ways in which catechins and related compounds might polymerize to yield condensed tannins. Of these, oxidative condensation3, either with or without enzyme catalysis, appears to be the most likely way in which polymerization occurs in vivo; for example, in plum leaves during the course of the growing season4, or in fruits on ripening5. Leuco-cyanidin (3,4,5,7,3′,4′-hexa-hydroxy-flavan) which is present in plum leaves and certain fruits and is the most widely occurring flavan in plant tissues6 should, on Hathway's hypothesis3, polymerize by a series of ‘head to tail’ condensations forming C–C bonds between C2′ of one molecule and C87 (or C6) of another. As the size of such a polymer increases it is obvious that the total number of phenolic hydroxyl groups will remain the same, and therefore their reactivity with analytical reagents should be roughly constant. Since, however, the most reactive positions of the phloroglucinol (A) ring of the flavan is involved, the reactivity of the polymers to analytical reagents which only substitute in this ring should be much reduced. The same reasoning of course applies to acid-catalysed polymerizations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Freudenberg, K., and Weinger, K., in The Chemistry of Flavonoid Compounds, edit. by Geissman, T. A., 197 (Pergamon Press, Oxford, 1962).
Hergert, H., The Chemistry of Flavonoid Compounds, 553.
Hathway, D. E., in Wood Extractives, edit. by Hillis, W. E., 191 (Academic Press, New York, 1962).
Hillis, W. E., and Swain, T., J. Sci. Food Agric., 10, 135 (1959).
Swain, T., and Goldstein, J. L., Proc. First Intern. Cong. Food. Sci. and Technol. (in the press).
Bate-Smith, E. C., Sci. Proc. Roy. Dublin Soc., 27, 165 (1956).
Hillis, W. E., and Urbach, G., J. App. Chem., 9, 474 (1959).
Swain, T., and Hillis, W. E., J. Sci. Food Agric., 10, 63 (1959).
Hathway, D. E., Biochem. J., 70, 34 (1959).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
GOLDSTEIN, J., SWAIN, T. Methods for Determining the Degree of Polymerization of Flavans. Nature 198, 587–588 (1963). https://doi.org/10.1038/198587a0
Issue Date:
DOI: https://doi.org/10.1038/198587a0
This article is cited by
-
A new assay for quantifying brown algal phlorotannins and comparisons to previous methods
Journal of Chemical Ecology (1996)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.