Abstract
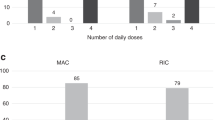
We compared outcome and toxicity in two paediatric groups undergoing SCT and treated with busulphan (BU) by the oral route of administration. One group receiving the standard dose of 1 mg/kg q.i.d. for a total of 16 doses was compared with age- and disease-matched patients receiving 2 mg/kg of BU b.i.d. for a total of eight doses. Seventy-two patients from two Swedish paediatric transplantation centres were included; one centre used a standard q.i.d. administration (n=37) and the second centre used a b.i.d. administration setting (n=35). Our primary objective was to determine the incidence of veno-occlusive disease (VOD), graft-versus-host disease (GVHD), relapse frequency and transplant-related mortality in both cohorts. A total of 17 autologous and 55 allogeneic transplantations was performed for malignant (n=47) and non-malignant (n=25) diseases in the two centres during the period 1990–2005. No significant difference in the incidence of VOD, graft rejection, GVHD, relapse rate or overall survival was observed between the two centres. The clinical outcome of SCT for paediatric patients conditioned with oral BU at a dose of 2 mg/kg for eight doses is comparable to that found for children conditioned using the standard regimen given 1 mg/kg q.i.d. for 16 doses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Hassan M, Ehrsson H . Urinary metabolites of busulfan in the rat. Drug Metab Dispos 1987; 15: 399–402.
Srivastava A, Poonkuzhali B, Shaji RV, George B, Mathews V, Chandy M et al. Glutathione S-transferase M1 polymorphism: a risk factor for hepatic venoocclussive disease in bone marrow transplantation. Blood 2004; 104: 1574–1577.
Kusama M, Kubota T, Matsukura Y, Matsuno K, Ogawa S, Kanda Y et al. Influence of glutathione S-transferase A1 polymorphism on the pharmacokinetics of busulfan. Clin Chim Acta 2006; 368: 93–98.
Hassan M, Ljungman P, Bolme P, Ringdèn O, Syrucková Z, Békàssy A et al. Busulfan bioavaliability. Blood 1994; 84: 2144–2150.
Bullock JM, Smith PF, Booker BM, Loughner J, Capozzi D, McCarthy Jr PL et al. Development of a pharmacokinetic and Bayesian optimal sampling model for individualization of oral busulfan in hematopoietic stem cell transplantation. Ther Drug Monit 2006; 28: 62–66.
McCune JS, Gibbs JP, Slattery JT . Plasma concentration monitoring of busulfan: does it improve clinical outcome? Clin Pharmacokinet 2000; 39: 155–165.
Bolinger AM, Zangwill AB, Slattery JT, Risler LJ, Sultan DH, Glidden DV et al. Target dose adjustment of busulfan in pediatric patients undergoing bone marrow transplantation. Bone Marrow Transplant 2001; 28: 1013–1018.
Sandström M, Karlsson MO, Ljungman P, Hassan Z, Jonsson EN, Nilsson C et al. Population pharmacokinetic analysis resulting in a tool for dose individualization of busulphan in bone marrow transplantation recipients. Bone Marrow Transplant 2001; 28: 657–664.
Hassan M, Fasth A, Gerritsen B, Haraldsson A, Syrucková Z, van den Berg H et al. Busulphan kinetics and limited sampling model in children with leukemia and inherited disorders. Bone Marrow Transplant 1996; 18: 843–850.
Bolinger AM, Zangwill AB, Slattery JT, Glidden D, DeSantes K, Heyn L et al. An evaluation of engraftment, toxicity and busulfan concentration in children receiving bone marrow transplantation for leukemia or genetic disease. Bone Marrow Transplant 2000; 25: 925–930.
Slattery JT, Clift RA, Buckner CD, Radich J, Storer B, Bensinger WI et al. Marrow transplantation for chronic myeloid leukemia: the influence of plasma busulfan levels on the outcome of transplantation. Blood 1997; 89: 3055–3060.
Slattery JT, Sanders JE, Buckner CD, Schaffer RL, Lambert KW, Langer FP et al. Graft-rejection and toxicity following bone marrow transplantation in relation to busulfan pharmacokinetics. Bone Marrow Transplant 1995; 16: 31–42.
Vassal G, Hartmann O, Benhamou E . Busulfan and veno-occlussive disease of the liver. Ann Intern Med 1990; 112: 881.
Grochow LB, Jones RJ, Brundrett RB, Braine HG, Chen TL, Saral R et al. Pharmacokinetics of busulfan: correlation with veno-occlusive disease in patients undergoing bone marrow transplantation. Cancer Chemother Pharmacol 1989; 25: 55–61.
Dix SP, Wingard JR, Mullins RE, Jerkunica I, Davidson TG, Gilmore CE et al. Association of busulfan area under the curve with veno-occlusive disease following BMT. Bone Marrow Transplant 1996; 17: 225–230.
Zwaveling J, Bredius RG, Cremers SC, Ball LM, Lankester AC, Teepe-Twiss IM et al. Intravenous busulfan in children prior to stem cell transplantation: study of pharmacokinetics in association with early clinical outcome and toxicity. Bone Marrow Transplant 2005; 35: 17–23.
Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation 1987; 44: 778–783.
Kaplan EL, Meier P . Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Mellgren K, Fasth A, Saalman R, Olausson M, Abrahamsson J . Liver transplantation after stem cell transplantation with the same living donor in a monozygotic twin with acute myeloid leukemia. Ann Hematol 2005; 84: 755–757.
Reiss U, Cowan M, McMillan A, Horn B . Hepatic venoocclusive disease in blood and bone marrow transplantation in children and young adults: incidence, risk factors, and outcome in a cohort of 241 patients. J Pediatr Hematol Oncol 2002; 24: 746–750.
Mc Donald GB, Sharma P, Matthews DE, Shulman HM, Thomas ED . Venocclusive disease of the liver after bone marrow transplantation: diagnosis, incidence, and predisposing factors. Hepatology 1984; 4: 116–122.
DeLeve LD . Glutathione defense in non-parenchymal cells. Semin Liver Dis 1998; 18: 403–413.
Jenke A, Freiberg-Richter J, Wilhelm S, Freund M, Renner UD, Bornhäuser M et al. Accidental busulfan overdose during conditioning for stem cell transplantation. Bone Marrow Transplant 2005; 35: 125–128.
Petersen FB, Sanders JE, Storb R, Bensinger WI, Clift RA, Buckner CD . Inadvertent administration of a greater-than-usual pre-marrow transplant dose of busulfan—report of a case. Transplantation 1988; 45: 821–822.
Stein J, Davidovitz M, Yaniv I, Ben-Ari J, Gamzu Z, Hoffer E et al. Accidental busulfan overdose: enhanced drug clearance with hemodialysis in a child with Wiskott–Aldrich syndrome. Bone Marrow Transplant 2001; 27: 551–553.
Hassan M, Ljungman P, Ringdén O, Hassan Z, Oberg G, Nilsson C et al. The effect of busulphan on the pharmacokinetics of cyclophoshamide and its 4-hydroxy metabolite: time interval influence on therapeutic efficacy and therapy-related toxicity. Bone Marrow Transplant 2000; 25: 915–924.
Schuler US, Renner UD, Kroschinsky F, Johne C, Jenke A, Naumann R et al. Intravenous busulphan for conditioning before autologous or allogeneic human blood stem cell transplantation. Br J Haematol 2001; 114: 944–950.
Takama H, Tanaka H, Nakashima D, Ueda R, Takaue Y . Population pharmacokinetics of intravenous busulfan in patients undergoing hematopoietic stem cell population. Bone Marrow Transplant 2006; 37: 345–351.
Acknowledgements
This study was supported by grants from the Swedish Cancer foundation as well as the Swedish Children Cancer Society.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mellgren, K., Nilsson, C., Fasth, A. et al. Safe administration of oral BU twice daily during conditioning for stem cell transplantation in a paediatric population: a comparative study between the standard 4-dose and a 2-dose regimen. Bone Marrow Transplant 41, 621–625 (2008). https://doi.org/10.1038/sj.bmt.1705947
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705947
Keywords
This article is cited by
-
The pharmacokinetics and safety of twice daily i.v. BU during conditioning in pediatric allo-SCT recipients
Bone Marrow Transplantation (2013)