Abstract
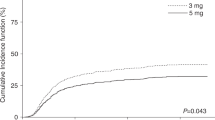
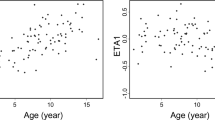
Cyclosporine A (CsA) therapy based on 2-h concentrations (C2) after oral administration has demonstrated low acute rejection rates after solid organ transplantation. We analysed the correlation between C2 and trough (C0) levels of oral CsA therapy in samples obtained twice in consecutive weeks from 58 patients during their first admission for allogeneic haematopoietic stem cell transplantation. Also 8-h concentration curves were obtained from 23 patients. The mean (range) CsA dose was 332 (167–763) and 255 (113–575) mg/day for patients with matched unrelated donor (MUD) and human leukocyte antigen identical sibling donor (Sib), respectively. Median (range) C0 and C2 were 254 (145–332) and 898 (419–1466) ng/ml in MUD patients, and 130 (93–265) and 554 (196–988) ng/ml in Sib patients. In MUD patients with either aGVHD grade
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cantarovich M, Fitchett D, Latter DA . Cyclosporine trough levels, acute rejection, and renal dysfunction after heart transplantation. Transplantation 1995; 59: 444–447.
Cole E, Keown P, Landsberg D, Halloran P, Shoker A, Rush D et al. Safety and tolerability of cyclosporine and cyclosporine microemulsion during 18 months of follow-up in stable renal transplant recipients: a report of the Canadian Neoral Renal Study Group. Transplantation 1998; 65: 505–510.
Halloran PF, Helms LM, Kung L, Noujaim J . The temporal profile of calcineurin inhibition by cyclosporine in vivo. Transplantation 1999; 68: 1356–1361.
Cantarovich M, Elstein E, de Varennes B, Barkun JS . Clinical benefit of neoral dose monitoring with cyclosporine 2-hr post-dose levels compared with trough levels in stable heart transplant patients. Transplantation 1999; 68: 1839–1842.
Morris RG, Russ GR, Cervelli MJ, Juneja R, McDonald SP, Mathew TH . Comparison of trough, 2-hour, and limited AUC blood sampling for monitoring cyclosporin (Neoral) at day 7 post-renal transplantation and incidence of rejection in the first month. Ther Drug Monit 2002; 24: 479–486.
Pescovitz MD, Barbeito R . Two-hour post-dose cyclosporine level is a better predictor than trough level of acute rejection of renal allografts. Clin Transplant 2002; 16: 378–382.
Caforio AL, Tona F, Piaserico S, Gambino A, Feltrin AB, Fortina AB et al. C2 is superior to C0 as predictor of renal toxicity and rejection risk profile in stable heart transplant recipients. Transpl Int 2005; 18: 116–124.
Levy G, Burra P, Cavallari A, Duvoux C, Lake J, Mayer AD et al. Improved clinical outcomes for liver transplant recipients using cyclosporine monitoring based on 2-hr post-dose levels (C2). Transplantation 2002; 73: 953–959.
Langers P, Cremers SC, den Hartigh J, Veenendaal RA, ten Hove WR, Ringers J et al. Switching monitoring of emulsified cyclosporine from trough level to 2-hour level in stable liver transplant patients. Liver Transpl 2004; 10: 183–189.
Billaud EM . C2 versus C0 cyclosporine monitoring: still not the end. Transplantation 2005; 80: 542; author reply 543–544.
Nashan B, Bock A, Bosmans JL, Budde K, Fijter H, Jaques B et al. Use of Neoral C monitoring: a European consensus. Transpl Int 2005; 18: 768–778.
Olerup O, Zetterquist H . HLA-DR typing by PCR amplification with sequence-specific primers (PCR-SSP) in 2 h: an alternative to serological DR typing in clinical practice including donor–recipient matching in cadaveric transplantation. Tissue Antigens 1992; 39: 225–235.
Ringden O, Remberger M, Runde V, Bornhäuser M, Blau IW, Basara N et al. Peripheral blood stem cell transplantation from unrelated donors: a comparison with marrow transplantation. Blood 1999; 94: 455–464.
Ringden O, Remberger M, Persson U, Ljungman P, Aldener A, Andström E et al. Similar incidence of graft-versus-host disease using HLA-A, -B and -DR identical unrelated bone marrow donors as with HLA-identical siblings. Bone Marrow Transplant 1995; 15: 619–625.
Hentschke P, Barkholt L, Uzunel M, Mattsson J, Wersäll P, Pisa P et al. Low-intensity conditioning and hematopoietic stem cell transplantation in patients with renal and colon carcinoma. Bone Marrow Transplant 2003; 31: 253–261.
Remberger M, Svahn BM, Hentschke P, Löfgren C, Ringden O . Effect on cytokine release and graft-versus-host disease of different anti-T cell antibodies during conditioning for unrelated haematopoietic stem cell transplantation. Bone Marrow Transplant 1999; 24: 823–830.
Ringden O, Pihlstedt P, Markling L, Aschan J, Båryd I, Ljungman P et al. Prevention of graft-versus-host disease with T cell depletion or cyclosporin and methotrexate. A randomized trial in adult leukemic marrow recipients. Bone Marrow Transplant 1991; 7: 221–226.
Storb R, Deeg HJ, Whitehead J, Appelbaum F, Beatty P, Bensinger W et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med 1986; 314: 729–735.
Le Blanc K, Remberger M, Uzunel M, Mattsson J, Barkholt L, Ringden O . A comparison of nonmyeloablative and reduced-intensity conditioning for allogeneic stem-cell transplantation. Transplantation 2004; 78: 1014–1020.
McSweeney PA, Niederwieser D, Shizuru JA, Sandmaier BM, Molina AJ, Maloney DG et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood 2001; 97: 3390–3400.
Ringden O, Blom B, Collste H, Gahrton G, Groth CG, Grimfors G et al. Bone marrow transplantation for aplastic anemia and acute leukemia at Huddinge Hospital. Scand J Urol Nephrol Suppl 1981; 64: 238–245.
Ringden O . Management of graft-versus-host disease. Eur J Haematol 1993; 51: 1–12.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974; 18: 295–304.
Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med 1980; 69: 204–217.
Ljungman P, Griffiths P, Paya C . Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis 2002; 34: 1094–1097.
MacMillan ML, Weisdorf DJ, Wagner JE, DeFor TE, Bums LJ, Ramsay NK et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant 2002; 8: 387–394.
Ringden O, Remberger M, Carlens S, Hägglund H, Mattsson J, Aschan J et al. Low incidence of acute graft-versus-host disease, using unrelated HLA-A-, HLA-B-, and HLA-DR-compatible donors and conditioning, including anti-T-cell antibodies. Transplantation 1998; 66: 620–625.
Hendriks MP, Blijlevens NM, Schattenberg AV, Burger DM, Donelly JP . Cyclosporine short infusion and C(2) monitoring in haematopoietic stem cell transplant recipients. Bone Marrow Transplant 2006; 38: 521–525.
Remberger M, Jaksch M, Uzunel M, Mattsson J . Serum levels of cytokines correlate to donor chimerism and acute graft-vs-host disease after haematopoietic stem cell transplantation. Eur J Haematol 2003; 70: 384–391.
Acknowledgements
This work has been supported by a grant from the National Network for Drug Development within The Foundation for Strategic Research, Sweden, and by the research funding of the Karolinska Institutet. We thank the patients and the staff at the Centre for allogeneic stem cell transplantation and the laboratory technicians at the Department of Clinical Pharmacology for compassionate and competent care of the patients, sample collection and analyse procedures. Novartis has generously contributed to cyclosporine A analyses by research funding, but has had no influence on the study protocol or preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barkholt, L., Remberger, M., Bodegård, H. et al. Cyclosporine A (CsA) 2-h concentrations vary between patients without correlation to graft-versus-host disease after allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant 40, 683–689 (2007). https://doi.org/10.1038/sj.bmt.1705788
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705788
Keywords
This article is cited by
-
Comparison of mycophenolate mofetil and calcineurin inhibitor versus calcineurin inhibitor-based graft-versus-host-disease prophylaxis for matched unrelated donor transplant in acute myeloid leukemia. A study from the ALWP of the EBMT
Bone Marrow Transplantation (2021)
-
Pharmacokinetics, Pharmacodynamics and Pharmacogenomics of Immunosuppressants in Allogeneic Haematopoietic Cell Transplantation: Part I
Clinical Pharmacokinetics (2016)
-
CsA 2-h concentration correlates best with area under the concentration–time curve after allo-SCT compared with trough CsA
Bone Marrow Transplantation (2012)
-
Links Between Cyclosporin Exposure in Tissues and Graft-Versus-Host Disease in Pediatric Bone Marrow Transplantation: Analysis by a PBPK Model
Pharmaceutical Research (2011)
-
CsA exposure is associated with acute GVHD and relapse in children after SCT
Bone Marrow Transplantation (2010)