Abstract
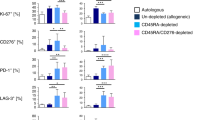
Selective depletion of alloreactive T cells from stem-cell allografts should abrogate graft-versus-host disease while preserving beneficial T cell specificities to facilitate engraftment and immune reconstitution. We therefore explored a refined immunomagnetic separation strategy to effectively deplete alloreactive donor lymphocytes expressing the activation antigen CD69 upon stimulation, and examined the retainment of antiviral, antileukemic, and immunoregulatory T cells. In addition to the CD69high T cell fraction, our studies retrieved two T cell subsets based on residual CD69 expression. Whereas, truly CD69neg cells were devoid of detectable alloresponses to original stimulators, CD69-low (CD69low)-expressing T cells elicited significant residual alloreactivity upon restimulation. In interferon-γ enzyme linked immunospot assays, anti-cytomegalovirus and anti-Epstein–Barr virus responses were preserved at significant numbers among CD69neg T lymphocytes. Accordingly, T cells recognizing the leukemia-associated Wilm's tumor-1 antigen were still detectable in the CD69neg subset. However, antiviral and antileukemic specificities were also consistently found within CD69low T cells, suggesting that memory-type donor T cells were partially captured due to residual CD69 expression. Finally, CD4+CD25+ Foxp3+ immunoregulatory T cells did not upregulate CD69 upon allogeneic stimulation. Our data suggest that CD69-mediated removal of alloreactivity can result in efficient allodepletion, but may partially affect the persistence of antiviral and antileukemic donor memory specificities captured among CD69low-expressing lymphocytes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Martin PJ . Donor CD8 cells prevent allogeneic marrow graft rejection in mice: potential implications for marrow transplantation in humans. J Exp Med 1993; 178: 703–712.
Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha ME, Greenberg PD . Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T-cell clones. Science 1992; 257: 238–241.
Kolb HJ, Mittermuller J, Clemm C, Holler E, Ledderose G, Brehm G et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood 1990; 76: 2462–2465.
Goldman JM, Gale RP, Horowitz MM, Biggs JC, Champlin RE, Gluckman E et al. Bone marrow transplantation for chronic myelogenous leukemia in chronic phase. Increased risk for relapse associated with T-cell depletion. Ann Intern Med 1988; 108: 806–814.
Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood 1990; 75: 555–562.
Ferrara JL, Levy R, Chao NJ . Pathophysiologic mechanisms of acute graft-vs-host disease. Biol Blood Marrow Transplant 1999; 5: 347–356.
Hartwig UF, Robbers M, Wickenhauser C, Huber C . Murine acute graft-versus-host disease can be prevented by depletion of alloreactive T lymphocytes using activation-induced cell death. Blood 2002; 99: 3041–3049.
Yang YG, Qi J, Wang MG, Sykes M . Donor-derived interferon gamma separates graft-versus-leukemia effects and graft-versus-host disease induced by donor CD8 T cells. Blood 2002; 99: 4207–4215.
Slavin S, Morecki S, Weiss L, Or R . Immunotherapy of hematologic malignancies and metastatic solid tumors in experimental animals and man. Crit Rev Oncol Hematol 2003; 46: 139–163.
Gao L, Yang TH, Tourdot S, Sadovnikova E, Hasserjian R, Stauss HJ . Allo-major histocompatibility complex-restricted cytotoxic T lymphocytes engraft in bone marrow transplant recipients without causing graft-versus-host disease. Blood 1999; 94: 2999–3006.
Barrett AJ, Malkovska V . Graft-versus-leukaemia: understanding and using the alloimmune response to treat haematological malignancies. Br J Haematol 1996; 93: 754–761.
Chen BJ, Cui X, Liu C, Chao NJ . Prevention of graft-versus-host disease while preserving graft-versus-leukemia effect after selective depletion of host-reactive T-cells by photodynamic cell purging process. Blood 2002; 99: 3083–3088.
Bonini C, Ferrari G, Verzeletti S, Servida P, Zappone E, Ruggieri L et al. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science 1997; 276: 1719–1724.
Mavroudis DA, Dermime S, Molldrem J, Jiang YZ, Raptis A, van Rhee F et al. Specific depletion of alloreactive T-cells in HLA-identical siblings: a method for separating graft-versus-host and graft-versus-leukaemia reactions. Br J Haematol 1998; 101: 565–570.
Andre-Schmutz I, Le Deist F, Hacein-Bey-Abina S, Vitetta E, Schindler J, Chedeville G et al. Immune reconstitution without graft-versus-host disease after haemopoietic stem-cell transplantation: a phase 1/2 study. Lancet 2002; 360: 130–137.
Amrolia PJ, Muccioli-Casadei G, Yvon E, Huls H, Sili U, Wieder ED et al. Selective depletion of donor alloreactive T-cells without loss of antiviral or antileukemic responses. Blood 2003; 102: 2292–2299.
Garderet L, Snell V, Przepiorka D, Schenk T, Lu JG, Marini F et al. Effective depletion of alloreactive lymphocytes from peripheral blood mononuclear cell preparations. Transplantation 1999; 67: 124–130.
Koh MB, Prentice HG, Lowdell MW . Selective removal of alloreactive cells from haematopoietic stem cell grafts: graft engineering for GVHD prophylaxis. Bone Marrow Transplant 1999; 23: 1071–1079.
Fehse B, Frerk O, Goldmann M, Bulduk M, Zander AR . Efficient depletion of alloreactive donor T lymphocytes based on expression of two activation-induced antigens (CD25 and CD69). Br J Hematol 2000; 109: 644–651.
Martins SL, St John LS, Champlin RE, Wieder ED, McMannis J, Molldrem JJ et al. Functional assessment and specific depletion of alloreactive human T cells using flow cytometry. Blood 2004; 104: 3429–3436.
Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S . Donor-type CD4(+) CD25(+) regulatory T-cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med 2002; 196: 389–399.
Salter RD, Howell DN, Cresswell P . Genes regulating HLA class I antigen expression in T-B lymphoblast hybrids. Immunogenetics 1985; 21: 235–246.
Britten CM, Meyer RG, Kreer T, Drexler I, Wolfel T, Herr W . The use of HLA-A*0201-transfected K562 as standard antigen-presenting cells for CD8(+) T lymphocytes in IFN-gamma ELISPOT assays. J Immunol Methods 2002; 259: 95–110.
Nagorsen D, Scheibenbogen C, Marincola FM, Letsch A, Keilholz U . Natural T-cell immunity against cancer. Clin Cancer Res 2003; 9: 4296–4303.
Gao L, Bellantuono I, Elsasser A, Marley SB, Gordon MY, Goldman JM et al. Selective elimination of leukemic CD34(+) progenitor cells by cytotoxic T lymphocytes specific for WT1. Blood 2000; 95: 2198–2203.
Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S et al. CD4+ CD25+ regulatory T-cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med 2003; 9: 1144–1150.
Ng WF, Duggan PJ, Ponchel F, Matarese G, Lombardi G, Edwards AD et al. Human CD4(+) CD25(+) cells: a naturally occurring population of regulatory T-cells. Blood 2001; 98: 2736–2744.
Levings MK, Sangregorio R, Roncarolo MG . Human cd25(+)cd4(+) t regulatory cells suppress naive and memory T-cell proliferation and can be expanded in vitro without loss of function. J Exp Med 2001; 193: 1295–1302.
Hori S, Nomura T, Sakaguchi S . Control of regulatory T cell development by the transcription factor Foxp3. Science 2003; 299: 1057–1061.
Gribben JG, Guinan EC, Boussiotis VA, Ke XY, Linsley L, Sieff C et al. Complete blockade of B7 family-mediated costimulation is necessary to induce human alloantigen-specific anergy: a method to ameliorate graft-versus-host disease and extend the donor pool. Blood 1996; 87: 4887–4893.
Craston R, Koh M, Mc Dermott A, Ray N, Prentice HG, Lowdell MW . Temporal dynamics of CD69 expression on lymphoid cells. J Immunol Methods 1997; 209: 37–45.
Riddell SR, Greenberg PD . T-cell therapy of human CMV and EBV infection in immunocompromised hosts. Rev Med Virol 1997; 7: 181–192.
Kolb HJ, Schmid C, Barrett AJ, Schendel DJ . Graft-versus-leukemia reactions in allogeneic chimeras. Blood 2004; 103: 767–776.
Inoue K, Ogawa H, Sonoda Y, Kimura T, Sakabe H, Oka Y et al. Aberrant overexpression of the Wilms tumor gene (WT1) in human leukemia. Blood 1997; 89: 1405–1412.
Dunn HS, Douglas JH, Ghansekar SA, Stepick-Biek P, Lewis DB, Maecker HT . Dynamics of CD4 and CD8 T-cell responses to cytomegalovirus in healthy human donors. J Infect Dis 2002; 186: 15–22.
Tan LC, Mowat AG, Fazou C, Rostron T, Roskell H, Dunbar PR et al. Specificity of T-cells in synovial fluid: high frequencies of CD8+ T-cells that are specific for certain viral epitopes. Arthritis Res 2000; 2: 154–164.
Wood KJ, Sakaguchi S . Regulatory T-cells in transplantation tolerance. Nat Rev Immunol 2003; 3: 199–210.
Shevach EM . Certified professionals: CD4(+) CD25(+) suppressor T-cells. J Exp Med 2001; 193: 41–46.
Davies JK, Koh MB, Lowdell M . Antiviral immunity and T-regulatory cell function are retained after selective alloreactive T-cell depletion in both the HLA-identical and HLA-mismatched settings. Biol Blood Marrow Transplant 2004; 10: 259–268.
Riddell SR, Greenberg PD . The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand human antigen-specific T-cells. J Immunol Methods 1990; 128: 189–201.
Rauser G, Einsele H, Sinzger C, Wernet D, Kuntz G, Assenmacher M et al. Rapid generation of combined CMV specific CD4+ and CD8+ cell lines for adoptive transfer into recipients of allogeneic stem cell transplants. Blood 2004; 103: 3565–3572.
Acknowledgements
This work was supported by grants from the German Cancer Aid (Deutsche Krebshilfe; Projects 70-3344-IIA and IVB), and by a grant from the MAIFOR program of Mainz University School of Medicine.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hartwig, U., Nonn, M., Khan, S. et al. Depletion of alloreactive T cells via CD69: implications on antiviral, antileukemic and immunoregulatory T lymphocytes. Bone Marrow Transplant 37, 297–305 (2006). https://doi.org/10.1038/sj.bmt.1705238
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705238
Keywords
This article is cited by
-
Translational Implications for Off-the-shelf Immune Cells Expressing Chimeric Antigen Receptors
Molecular Therapy (2016)
-
Haploidentical HSCT: a 15-year experience at San Raffaele
Bone Marrow Transplantation (2015)
-
Accelerating immune reconstitution after hematopoietic stem cell transplantation
Clinical & Translational Immunology (2014)
-
The humanized anti-HLA-DR moAb, IMMU-114, depletes APCs and reduces alloreactive T cells: implications for preventing GVHD
Bone Marrow Transplantation (2012)