Summary:
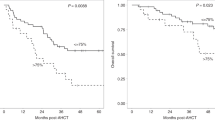
The purpose of this study was to evaluate pre-transplant T-cell status in autologous hematopoietic progenitor-cell transplantation (HPCT) recipients. Between 1999 and 2002 we prospectively enrolled 85 autologous HPCT recipients with solid tumors (N=50) or hematological malignancies (n=35). Patient diagnoses included breast cancer (N=49), non-Hodgkin's lymphoma (N=20), myeloma (N=11), Hodgkin's disease (N=3), germ-cell tumor (N=1) and amyloidosis (N=1). Levels of CD3, CD4, CD8, memory and naïve CD4, and CD8 T-cell subsets were analyzed before autologous HPCT. Autologous HPCT recipients presented with lower pre-transplant counts of CD3, CD4, but not CD8 T cells, as compared to healthy controls. Pre-transplant CD4 T-cell levels correlated with progression-free survival (PFS) (P=0.002) and overall survival (OS) (P=0.05), in patients with hematologic malignancies (P=0.02) and breast cancer (P=0.04). Specifically, pre-transplant memory CD4 + CD45RA − CD62L − T-cell levels correlated with PFS (P=0.01). The prognostic effects of pre-transplant CD4 and CD4 + CD45RA − CD62L − T cells were independent of tumor diagnosis, tumor stage, tumor sensitivity, and, for breast cancer patients, Her2 / neu status. Our results suggest that pre-transplant CD4 T-cell status, specifically CD4 + CD45RA − CD62L − memory T cells, correlates with the outcome of autologous HPCT recipients. These observations suggest the feasibility of prospective identification of those patients at higher risk of relapse, based on their immune status.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kolb HJ, Mittermuller J, Clemm C et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood 1990; 76: 2462–2465.
Kolb HJ, Schattenberg A, Goldman JM et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood 1995; 86: 2041–2050.
Goldman JM, Gale RP, Horowitz MM et al. Bone marrow transplantation for chronic myelogenous leukemia in chronic phase. Increased risk for relapse associated with T-cell depletion. Ann Intern Med 1988; 108: 806–814.
Apperley JF, Mauro FR, Gregory W et al. Bone marrow transplantation for chronic myeloid leukaemia in first chronic phase: Importance of a graft-versus-leukemia effect. Br J Haematol 1988; 69: 239–245.
Martin PJ, Clift RA, Fisher LD et al. HLA-identical marrow transplantation during accelerated-phase chronic myelogenous leukemia: analysis of survival and remission duration. Blood 1988; 72: 1978–1984.
Mackall CL . T-cell immunodeficiency following cytotoxic antineoplastic therapy: a review. Stem Cells 2000; 18: 10–18.
Mackall CL, Fleisher TA, Brown MR et al. Lymphocyte depletion during treatment with intensive chemotherapy for cancer. Blood 1994; 84: 2221–2228.
Mackall CL, Stein D, Fleisher TA et al. Prolonged CD4 depletion after sequential autologous peripheral blood progenitor cell infusion in children and young adults. Blood 2000; 96: 754–762.
Blanck G . Mutations and regulatory anomalies effecting tumor cell immune functions. Cancer Immunol Immunother 2004; 53: 1–16.
Peters WP, Eder JP, Henner WD et al. High-dose combination alkylating agents with autologous bone marrow support: a phase 1 trial. J Clin Oncol 1986; 4: 646–654.
Gaspard MH, Maraninchi D, Stoppa AM et al. Intensive chemotherapy with high doses of BCNU, etoposide, cytosine arabinoside, and melphalan (BEAM) followed by autologous bone marrow transplantation: toxicity and antitumor activity in 26 patients with poor-risk malignancies. Cancer Chemother Pharmacol 1988; 22: 256–262.
Barlogie B, Alexanian R, Dicke KA et al. High-dose chemoradiotherapy and autologous bone marrow transplantation for resistant multiple myeloma. Blood 1987; 70: 869–872.
Nieto Y, Vredenburgh J, Shpall EJ et al. Pilot study of concurrent administration of trastuzumab with high-dose cyclophosphamide, cisplatin, and BCNU, with autologous hematopoietic progenitor-cell support, in patients with advanced HER2-positive breast cancer. Clin Cancer Res 2004; 10: 7136–7143.
Nieto Y, Shpall EJ, Bearman SI et al. Phase I and pharmacokinetic study of docetaxel combined with melphalan and carboplatin, with autologous hematopoietic progenitor cell support, in patients with advanced refractory malignancies. Biol Blood Marrow Transplant 2005; 11: 297–306.
Roederer M, De Rosa SC, Watanabe N, Herzenberg LA . Dynamics of fine T-cell subsets during HIV disease and after thymic ablation by mediastinal irradiation. Semin Immunol 1997; 9: 389–396.
Watanabe N, De Rosa SC, Cmelak A et al. Long-term depletion of naïve T cells in patients treated for Hodgkin's disease. Blood 1997; 90: 3662–3672.
De Rosa SC, Herzenberg LA, Herzenberg LA, Roederer M . 11-color, 13-parameter flow cytometry: identification of human naïve T cells by phenotype, function, and T-cell receptor diversity. Nat Med 2001; 7: 245–248.
Kaplan EL, Meier P . Nonparametric estimation from incomplete observations. JAMA 1958; 53: 457–481.
Peto R, Peto J . Regression models and life tables. J R Stat Soc A 1972; 135: 185–188.
Cox DR . Regression models and life tables. J R Stat Soc B 1972; 34: 187–202.
Guillaume T, Rubinstein DB, Symann M . Immune reconstitution and immunotherapy after autologous hematopoietic stem cell transplantation. Blood 1998; 92: 1471–1490.
Porrata LF, Litzow MR, Markovic SN . Immune reconstitution after autologous hematopoietic stem cell transplantation. Mayo Clin Proc 2001; 76: 407–412.
Porrata LF, Gertz MA, Inwards DJ et al. Early lymphocyte recovery predicts superior survival after autologous hematopoietic stem cell transplantation in multiple myeloma or non-Hodgkin lymphoma. Blood 2001; 98: 579–585.
Porrata LF, Ingle JN, Litzow MR et al. Prolonged survival associated with early lymphocyte recovery after autologous hematopoietic stem cell transplantation for patients with metastatic breast cancer. Bone Marrow Transplant 2001; 28: 865–871.
Porrata LF, Inwards DJ, Micallef IN et al. Early lymphocyte recovery post-autologous haematopoietic stem cell transplantation is associated with better survival in Hodgkin's disease. Br J Haematol 2002; 117: 629–633.
Porrata LF, Litzow MR, Tefferi A et al. Early lymphocyte recovery is a predictive factor for prolonged survival after autologous hematopoietic stem cell transplantation for acute myelogenous leukemia. Leukemia 2002; 16: 1311–1318.
Nieto Y, Shpall EJ, McNiece IK et al. Prognostic evaluation of the early lymphocyte recovery in patients with advanced metastatic and non-metastastic breast cancer receiving high-dose chemotherapy with an autologous stem-cell transplant. Clin Cancer Res 2004; 10: 5076–5086.
Porrata LF, Litzow MR, Inwards DJ et al. Infused peripheral blood autograft absolute lymphocyte count correlates with day 15 absolute lymphocyte count and clinical outcome after autologous peripheral hematopoietic stem cell transplantation in non-Hodgkin's lymphoma. Bone Marrow Transplant 2004; 33: 291–298.
Porrata LF, Gertz MA, Geyer SM . The dose of infused lymphocytes in the autograft directly correlates with clinical outcome after autologous peripheral blood hematopoietic stem cell transplantation in multiple myeloma. Leukemia 2004; 18: 1085–1092.
Kay NE, Leong TL, Bone N et al. Blood levels of immune cells predict survival in myeloma patients: results of an Eastern Cooperative Oncology Group phase 3 trial for newly diagnosed multiple myeloma patients. Blood 2001; 98: 23–28.
Kanegane H, Kasahara Y, Niida Y et al. Expression of L-selectin (CD62L) discriminates Th1- and Th2 like cytokine-producing memory CD4 + T cells. Immunol 1996; 87: 186–190.
Soiffer RJ, Murray C, Cochran K et al. Clinical and immunologic effects of prolonged infusion of low-dose recombinant interleukin-2 after autologous and T-cell-depleted allogeneic bone marrow transplantation. Blood 1992; 79: 517–526.
Nagler A, Ackerstein A, Or R et al. Immunotherapy with recombinant human interleukin-2 and recombinant interferon-alpha in lymphoma patients postautologous marrow or stem cell transplantation. Blood 1997; 89: 3951–3959.
Hamon MD, Prentice HG, Gottlieb DJ et al. Immunotherapy with interleukin 2 after ABMT in AML. Bone Marrow Transplant 1993; 11: 399–401.
Benyunes MC, Massumoto C, York A et al. Interleukin-2 with or without lymphokine-activated killer cells as consolidative immunotherapy after autologous bone marrow transplantation for acute myelogenous leukemia. Bone Marrow Transplant 1993; 12: 159–163.
Benyunes MC, Higuchi C, York A et al. Immunotherapy with interleukin 2 with or without lymphokine-activated killer cells after autologous bone marrow transplantation for malignant lymphoma: a feasibility trial. Bone Marrow Transplant 1995; 16: 283–288.
Lister J, Rybka WB, Donnenberg AD et al. Autologous peripheral blood stem cell transplantation and adoptive immunotherapy with activated natural killer cells in the immediate posttransplant period. Clin Cancer Res 1995; 1: 607–614.
Hsu FJ, Benike C, Fagnoni F et al. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med 1996; 2: 52–58.
Burns LJ, Weisdorf DJ, DeFor TE et al. Enhancement of the anti-tumor activity of a peripheral blood progenitor cell graft by mobilization with interleukin-2 plus granulocyte colony-stimulating factor in patients with advanced breast cancer. Exp Hematol 2000; 28: 96–103.
Sosman JA, Stiff P, Moss SM et al. Pilot trial of interleukin-2 with granulocyte colony-stimulating factor for the mobilization of progenitor cells in advanced breast cancer patients undergoing high-dose chemotherapy: Expansion of immune effectors within the stem-cell graft and post-stem-cell infusion. J Clin Oncol 2001; 19: 634–644.
Toh HC, McAfee SL, Sackstein R et al. High-dose cyclophosphamide + carboplatin and interleukin-2 (IL-2) activated autologous stem cell transplantation followed by maintenance IL-2 therapy in metastatic breast carcinoma – a phase II study. Bone Marrow Transplant 2000; 25: 19–24.
Gravis G, Viens P, Vey N et al. Pilot study of immunotherapy with interleukin-2 after autologous stem cell transplantation in advanced breast cancers. Anticancer Res 2000; 20: 3987–3991.
Perillo A, Pierelli L, Battaglia A et al. Administration of low-dose interleukin-2 plus G-CSF / EPO early after autologous PBSC transplantation: effects on immune recovery and NK activity in a prospective study in women with breast and ovarian cancer. Bone Marrow Transplant 2002; 30: 571–578.
Burns LJ, Weisdorf DJ, DeFor TE et al. IL-2-based immunotherapy after autologous transplantation for lymphoma and breast cancer induces immune activation and cytokine release: a phase I / II trial. Bone Marrow Transplant 2003; 32: 177–186.
Morse MA, Vredenburgh JJ, Lyerly HK . A comparative study of the generation of dendritic cells from mobilized peripheral blood progenitor cells of patients undergoing high-dose chemotherapy. J Hematother Stem Cell Res 1999; 8: 577–584.
de Gast GC, Vyth-Dreese FA, Nooijen W et al. Reinfusion of autologous lymphocytes with granulocyte-macrophage colony-stimulating factor induces rapid recovery of CD4 + and CD8 + T cells after high-dose chemotherapy for metastatic breast cancer. J Clin Oncol 2002; 20: 58–64.
Acknowledgements
We acknowledge the nurses and house staff of the University of Colorado Bone Marrow Transplant Program. We are grateful to our patients for their willingness to participate in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rosinski, S., McNiece, I., Shpall, E. et al. Prognostic analysis of pre-transplant peripheral T-cell levels in patients receiving an autologous hematopoietic progenitor-cell transplant. Bone Marrow Transplant 36, 425–430 (2005). https://doi.org/10.1038/sj.bmt.1705073
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705073
Keywords
This article is cited by
-
Early recovery of aggressive cytotoxic cells and improved immune resurgence with post-transplant immunotherapy for multiple myeloma
Bone Marrow Transplantation (2007)