Summary:
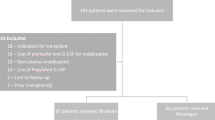
This prospective and randomized study was conducted to evaluate clinical and economic consequences of using granulocyte colony-stimulating factor (G-CSF) following autologous peripheral blood progenitor cell (PBPC) transplantation in children. Between January 1999 and December 2003, 117 patients underwent autologous PBPCT: 51 patients received G-CSF following PBPCT, while 66 patients did not receive G-CSF. Median time to absolute neutrophil count >0.5 × 109/l was 10 days in the treatment group and 11 days in the control group (P<0.009). The median time to platelets >20 × 109/l was 12 days in both groups (P=NS). The median time to platelets >50 × 109/l was 15 days in the G-CSF group and 14 days in the control group (P<0.005). In patients who received <5 × 106/kg CD34+ cells, the median time to platelets >20 × 109/l and >50 × 109/l was similar with or without G-CSF (12 and 15 days, respectively). Platelet transfusion requirements were lower in the control group (2 vs 3 U in G-CSF group). There was a trend towards higher total costs with G-CSF: 8146.82 euros and 7873.34 euros with and without G-CSF, respectively (P=0.1). Our data suggest that there is no indication of the standard application of G-CSF in children following PBPC transplantation. The only possible indication is the group of patients with a lower yield of CD34+ cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Shimazaki C, Oku N, Uchiyama H et al. Effect of granulocyte colony-stimulating factor on hematopoietic recovery after peripheral blood progenitor-cell transplantation. Bone Marrow Transplant 1994; 13: 271–275.
Demirer T, Ayli M, Dagli M et al. Influence of post-transplant recombinant human granulocyte colony-stimulating factor administration on peritransplant morbidity in patients undergoing autologous stem cell transplantation. Br J Haematol 2002; 118: 1104–1111.
Mc Quaker IG, Hunter AE, Pacey S et al. Low-dose filgrastim significantly enhances neutrophil recovery following autologous peripheral-blood stem-cell transplantation in patients with lymphoproliferative disorders: evidence for clinical and economic benefit. J Clin Oncol 1997; 15: 451–457.
Colby C, Mc Afee SL, Finkelstein DM et al. Early vs delayed administration of G-CSF following autologous peripheral blood stem cell transplantation. Bone Marrow Transplant 1998; 21: 1005–1010.
Bolwell BJ, Pohlman B, Andresen S et al. Delayed G-CSF after autologous progenitor cell transplantation: a prospective randomized trial. Bone Marrow Transplant 1998; 21: 369–373.
Klumpp TR, Mangan KF, Goldberg SL et al. Granulocyte colony stimulating factor accelerates neutrophil engraftment following peripheral-blood stem-cell transplantation: a prospective, randomized trial. J Clin Oncol 1995; 13: 1323–1327.
Tarella C, Castellino C, Locatelli F et al. G-CSF administration following peripheral blood progenitor cell (PBPC) autograft in lymphoid malignancies: evidence for clinical benefits and reduction of treatment costs. Bone Marrow Transplant 1998; 21: 401–407.
Kawano Y, Takaue Y, Mimaya J et al. Marginal benefit/disadvantage of granulocyte colony stimulating factor therapy after autologous blood stem cell transplantation in children: results of a prospective randomized trial. Blood 1998; 92: 4040–4046.
Díaz MA, Villa M, Alegre A et al. Collection and transplantation of peripheral blood progenitor cells mobilized by G-CSF alone in children with malignancies. Br J Haematol 1996; 94: 148–154.
Díaz MA, García-Sánchez F, Lillo R et al. Large-volume leukapheresis in pediatric patients: preapheresis peripheral blood CD34+ cell count predicts progenitor cell yield. Haematologica 1999; 84: 30–33.
González Vicent M, Madero L, Chamorro L et al. Comparative cost analysis of autologous peripheral blood progenitor cell and bone marrow transplantation in pediatric patients with malignancies. Haematologica 2001; 86: 1087–1094.
Sheridan WP, Wolf M, Lusk J et al. Granulocyte colony-stimulating factor and neutrophil recovery after high-dose chemotherapy and autologous bone marrow transplantation. Lancet 1989; 2: 891–895.
Madero L, Muñoz A, Díaz de Heredia A et al. G-CSF after autologous bone marrow transplantation for malignant diseases in children. Spanish Working Party for Bone Marrow Transplantation in children. Bone Marrow Transplant 1995; 15: 349–351.
Tarella C, Benedetti G, Caracciolo D et al. Both early and committed progenitors are more frequent in peripheral blood than in bone marrow during mobilization induced by high-dose chemotherapy+G-CSF. Br J Haematol 1995; 91: 535–543.
Ozer H, Armitage J, Benett CH et al. 2000 update of recommendations for the use of hematopoietic colony-stimulating factors: evidence-based, clinical practice guidelines. J Clin Oncol 2000; 18: 3558–3585.
Schaison G, Eden OB, Henze G et al. Recommendations on the use of colony-stimulating factors in children: conclusions of a European panel. Eur J Pediatr 1998; 157: 955–966.
Dallorso S, Rondelli R, Messina CH et al. Clinical benefits of granulocyte colony-stimulating factor therapy after hematopoietic stem cell transplant in children: results of a prospective randomized trial. Haematologica 2002; 87: 1274–1280.
González Vicent M, Madero L, Sevilla J, Díaz MA . Clinical and economic evaluation of using granulocyte colony-stimulating factor after autologous peripheral blood progenitor cell transplantation in children. Haematologica 2002; 87: 105–106.
Takaue Y, Watanabe T, Kawano Y et al. Sustained cytopenia in small children after leukapheresis for collection of peripheral blood stem cells. Vox Sang 1989; 57: 168–171.
Acknowledgements
This work was partially supported by ‘Fundación Oncohematología Pediátrica’.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
González-Vicent, M., Madero, L., Sevilla, J. et al. A prospective randomized study of clinical and economic consequences of using G-CSF following autologous peripheral blood progenitor cell (PBPC) transplantation in children. Bone Marrow Transplant 34, 1077–1081 (2004). https://doi.org/10.1038/sj.bmt.1704699
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704699
Keywords
This article is cited by
-
A systematic literature review of the efficacy, effectiveness, and safety of filgrastim
Supportive Care in Cancer (2018)
-
Cytokines following SCT: indications and controversies
Bone Marrow Transplantation (2008)
-
Granulocyte and erythropoietic stimulating proteins after high-dose chemotherapy for myeloma
Bone Marrow Transplantation (2007)