Abstract
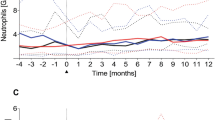
The effect of mixed chimerism on the pace of post-transplant immune reconstitution is unknown. Using flow cytometry, recall and neo-antigen vaccine responses, and T cell receptor recombination excision circle (TREC) quantification, we evaluated phenotypic and functional characteristics of T and B cells in nine patients following non-myeloablative, HLA-identical peripheral blood stem cell transplantation for chronic granulomatous disease. Engraftment of T cell, B cell, and myeloid lineages proceeded at similar paces within each patient, but engraftment kinetics segregated patients into two groups: adults, who became full donor T cell chimeras before 6 months (rapid engrafters) and children, who became full donor T cell chimeras after 6 months or not at all (slow engrafters). Quantitative B cell recovery was achieved by 6 weeks after transplantation in children, but was delayed until 1 year in adults. Early quantitative B cell recovery was not accompanied by an early humoral immune response to tetanus toxoid (TT). Emergence of TT-specific T cell responses coincided with naive T cell reconstitution, as measured by CD4/CD45RA T cell recovery and TREC quantification. These data suggest that immune reconstitution occurs faster in pediatric patients who have prolonged mixed hematopoietic chimerism compared to adults, who have rapid donor stem cell engraftment. Bone Marrow Transplantation (2001) 28, 463–471.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ljungman P, Wiklund-Hammarsten M, Duraj V et al. Response to tetanus toxoid immunization after allogeneic bone marrow transplantation J Infect Dis 1990 162: 496–500
Vance E, George S, Guinan EC et al. Comparison of multiple immunization schedules for Haemophilus influenzae type b-conjugate and tetanus toxoid vaccines following bone marrow transplantation Bone Marrow Transplant 1998 22: 735–741
Parkkali T, Olander RM, Ruutu T et al. A randomized comparison between early and late vaccination with tetanus toxoid vaccine after allogeneic BMT Bone Marrow Transplant 1997 19: 933–938
Small TN . Immunologic reconstitution following stem cell transplantation Curr Opin Hematol 1996 3: 461–465
Small TN, Avigan D, Dupont B et al. Immune reconstitution following T-cell depleted bone marrow transplantation: effect of age and posttransplant graft rejection prophylaxis Biol Blood Marrow Transplant 1997 3: 65–75
Keever CA, Small TN, Flomenberg N et al. Immune reconstitution following bone marrow transplantation: comparison of recipients of T-cell depleted marrow with recipients of conventional marrow grafts Blood 1989 73: 1340–1350
Ottinger HD, Beelen DW, Scheulen B et al. Improved immune reconstitution after allotransplantation of peripheral blood stem cells instead of bone marrow Blood 1996 88: 2775–2779
Martinez C, Urbano-Ispizua A, Rozman C et al. Immune reconstitution following allogeneic peripheral blood progenitor cell transplantation: comparison of recipients of positive CD34+ selected grafts with recipients of unmanipulated grafts Exp Hematol 1999 27: 561–568
Davison GM, Novitzky N, Kline A et al. Immune reconstitution after allogeneic bone marrow transplantation depleted of T cells Transplantation 2000 69: 1341–1347
Foot AB, Potter MN, Donaldson C et al. Immune reconstitution after BMT in children Bone Marrow Transplant 1993 11: 7–13
Godthelp BC, van Tol MJ, Vossen JM, van Den Elsen PJ . T-cell immune reconstitution in pediatric leukemia patients after allogeneic bone marrow transplantation with T-cell-depleted or unmanipulated grafts: evaluation of overall and antigen-specific T-cell repertoires Blood 1999 94: 4358–4369
Mackall CL, Fleisher TA, Brown MR et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy New Engl J Med 1995 332: 143–149
Weinberg K, Annett G, Kashyap A et al. The effect of thymic function on immunocompetence following bone marrow transplantation Biol Blood Marrow Transplant 1995 1: 18–23
Storek J, Witherspoon RP, Storb R . T cell reconstitution after bone marrow transplantation into adult patients does not resemble T cell development in early life Bone Marrow Transplant 1995 16: 413–425
Douek DC, Vescio RA, Betts MR et al. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution Lancet 2000 355: 1875–1881
Heitger A, Kern H, Mayerl D et al. Effective T cell regeneration following high-dose chemotherapy rescued with CD34+ cell enriched peripheral blood progenitor cells in children Bone Marrow Transplant 1999 23: 347–353
Gerritsen EJ, Van Tol MJ, Van't Veer MB et al. Clonal dysregulation of the antibody response to tetanus-toxoid after bone marrow transplantation Blood 1994 84: 4374–4382
Gerritsen EJ, van Tol MJ, Lankester AC et al. Immunoglobulin levels and monoclonal gammopathies in children after bone marrow transplantation Blood 1993 82: 3493–3502
Aucouturier P, Barra A, Intrator L et al. Long lasting IgG subclass and antibacterial polysaccharide antibody deficiency after allogeneic bone marrow transplantation Blood 1987 70: 779–785
Kelsey SM, Lowdell MW, Newland AC . IgG subclass levels and immune reconstitution after T cell-depleted allogeneic bone marrow transplantation Clin Exp Immunol 1990 80: 409–412
Velardi A, Cucciaioni S, Terenzi A et al. Acquisition of Ig isotype diversity after bone marrow transplantation in adults. A recapitulation of normal B cell ontogeny J Immunol 1988 141: 815–820
Storek J, Saxon A . Reconstitution of B cell immunity following bone marrow transplantation Bone Marrow Transplant 1992 9: 395–408
Nagler A, Slavin S, Varadi G et al. Allogeneic peripheral blood stem cell transplantation using a fludarabine-based low intensity conditioning regimen for malignant lymphoma Bone Marrow Transplant 2000 25: 1021–1028
Childs R, Clave E, Contentin N et al. Engraftment kinetics after nonmyeloablative allogeneic peripheral blood stem cell transplantation: full donor T-cell chimerism precedes alloimmune responses Blood 1999 94: 3234–3241
Horwitz ME, Barrett AJ, Brown MR et al. Treatment of chronic granulomatous disease with nonmyeloablative conditioning and a T-cell-depleted hematopoietic allograft New Engl J Med 2001 344: 881–888
van Leeuwen, van Tol MJ, Joosten AM et al. Persistence of host-type hematopoiesis after allogeneic bone marrow transplantation for leukemia is significantly related to the recipient's age and/or the conditioning regimen, but it is not associated with an increased risk of relapse Blood 1994 83: 3059–3067
Haynes BF, Markert, ML, Sempowski GD et al. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection Ann Rev Immunol 2000 18: 529–560
Weinberg K, Blazar BR, Wagner J et al. Factors affecting thymic function after allogeneic hematopoietic stem cell transplantation Blood 2001 97: 1458–1466
de Vries E, van Tol MJ, van den Bergh RL et al. Reconstitution of lymphocyte subpopulations after paediatric bone marrow transplantation Bone Marrow Transplant 2000 25: 267–275
Shenoy S, Mohanakumar T, Todd G et al. Immune reconstitution following allogeneic peripheral blood stem cell transplants Bone Marrow Transplant 1999 23: 335–346
Behringer D, Bertz H, Schmoor C et al. Quantitative lymphocyte subset reconstitution after allogeneic hematopoietic transplantation from matched related donors with CD34+ selected PBPC grafts unselected PBPC grafts or BM grafts Bone Marrow Transplant 1999 24: 295–302
Denny T, Yogev R, Gelman R et al. Lymphocyte subsets in healthy children during the first 5 years of life JAMA 1992 267: 1484–1488
Storek J, Witherspoon RP, Webb D, Storb R . Lack of B cells precursors in marrow transplant recipients with chronic graft-versus-host disease Am J Hematol 1996 52: 82–89
Eisner MD, August CS . Impact of donor and recipient characteristics on the development of acute and chronic graft-versus-host disease following pediatric bone marrow transplantation Bone Marrow Transplant 1995 15: 663–668
Acknowledgements
This work was supported in part by the Clinical Research Training Program, a partnership between the Foundation for the NIH and Pfizer Inc, Leukemia and Lymphoma Society of America translational research grant 6540–00, and AMFAR grant 02680–28-RGV.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Savage, W., Bleesing, J., Douek, D. et al. Lymphocyte reconstitution following non-myeloablative hematopoietic stem cell transplantation follows two patterns depending on age and donor/recipient chimerism. Bone Marrow Transplant 28, 463–471 (2001). https://doi.org/10.1038/sj.bmt.1703176
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1703176
Keywords
This article is cited by
-
Pharmacokinetics, Pharmacodynamics and Pharmacogenomics of Immunosuppressants in Allogeneic Haematopoietic Cell Transplantation: Part I
Clinical Pharmacokinetics (2016)
-
Optimizing drug therapy in pediatric SCT: Focus on pharmacokinetics
Bone Marrow Transplantation (2015)
-
Immune reconstitution after allogeneic stem cell transplantation with reduced-intensity conditioning regimens
Leukemia (2007)
-
No recovery of T-cell receptor excision circles (TRECs) after non-myeloablative allogeneic hematopoietic stem cell transplantation is correlated with the onset of GvHD
Journal of Applied Genetics (2007)
-
A comparison of T-, B- and NK-cell reconstitution following conventional or nonmyeloablative conditioning and transplantation with bone marrow or peripheral blood stem cells from human leucocyte antigen identical sibling donors
Bone Marrow Transplantation (2003)