Abstract
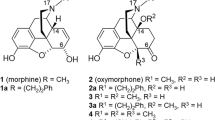
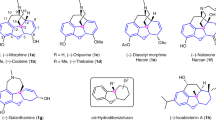
THE morphine molecule contains five asymmetric carbon atoms and five interlocking ring systems, and constitutes a complicated three-dimensional structure the stereochemistry of which has recently attracted considerable interest. The initial suggestions of Schöpf1 concerning the relative orientations at the asymmetric carbons 5, 9, 13 and 14 were extended by Fieser and Fieser2 to include carbon 6, and in terms of these deductions morphine has structure I or its mirror image. Further evidence leading independently to the same conclusion has been found3 by Dr. H. L. Holmes of the Riker Institute, Los Angeles, and confirmation of the relative configurations shown at carbons 5, 6, 9 and 13 has been provided by Rapoport and Payne4. However, up to the present, it has not been possible to decide whether structure I represents morphine or its enantiomorph.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Schöpf, C., Ann., 452, 211 (1927). Schöpf, C., and Pfeifer, T., Ann., 483, 157 (1930).
Fieser, L. F., and Fieser, M., “Natural Products Related to Phenanthrene”, 24 (3rd edit., Reinhold, New York, 1949).
Holmes, H. L. (unpublished).
Rapoport, H., and Payne, G. B., J. Org. Chem., 15, 1093 (1950). Rapoport, H., and Payne, G. B., J. Amer. Chem. Soc. (in the press).
Clough, G. W., J. Chem. Soc., 113, 526 (1918).
Leithe, W., Ber., 63, 1498 (1930).
Leithe, W., Ber., 63, 2343 (1930); 64, 2827 (1931); 67, 1261 (1934).
Bijvoet, J. M., Peerdeman, A. F., and van Bommel, A. J., Nature, 168, 271 (1951).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BICK, I. Absolute Stereochemical Configuration of Morphine. Nature 169, 755–756 (1952). https://doi.org/10.1038/169755a0
Issue Date:
DOI: https://doi.org/10.1038/169755a0
This article is cited by
-
Stereochemistry of Certain Analgesics
Nature (1954)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.