Abstract
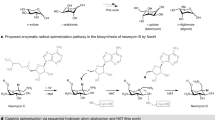
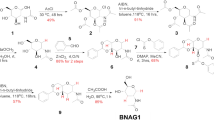
DURING recent years, a series of papers from this laboratory have shown that derivatives of glucose may be converted smoothly into derivatives of altrose, galactose and gulose by means of optical inversion within the molecule1. In each case the key substance to such a conversion has been an anhydro-compound of the ethylene oxide type in which the ring is broken under the influence of alkali. For example, 2: 3-anhydro 4: 6-benzylidene α-methylalloside or 2: 3-anhydro 4: 6-benzylidene α-methylmannoside, in our experience, invariably yield derivatives of altrose when treated with alcoholic caustic potash or sodium methoxide solution.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Mathers and Robertson, J. Chem. Soc., 1076 (1933); Robertson and Griffith, J. Chem. Soc., 1193 (1935); Oldham and Robertson, J. Chem. Soc., 685 (1935).
Ber., 53, 541 (1920).
Ber., 59, 714 (1926).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
ROBERTSON, G., MYERS, W. & TETLOW, W. “Methyl Epiglucosamine” and 2-Amino α-Methyl-altroside. Nature 142, 1076–1077 (1938). https://doi.org/10.1038/1421076b0
Issue Date:
DOI: https://doi.org/10.1038/1421076b0
This article is cited by
-
New Acetylated Derivatives of Amino Sugars
Nature (1939)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.