Abstract
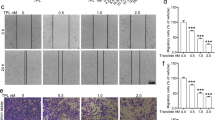
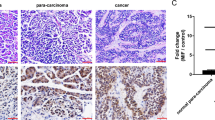
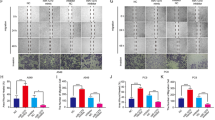
Cancer cell invasion is one of the crucial events in local spreading, growth and metastasis of tumors. The present study investigates the mechanism underlying the anti-invasive action of the chemotherapeutic cisplatin. In human cervical carcinoma cells (HeLa), cisplatin caused a time- and concentration-dependent suppression of cell invasion through Matrigel. Inhibition of invasion was accompanied by upregulation of tissue inhibitor of matrix metalloproteinases-1 (TIMP-1), whereas levels of matrix metalloproteinase-2 (MMP-2), MMP-9 and TIMP-2 remained unchanged. Cisplatin's effects on TIMP-1 expression and invasion were associated with phosphorylations of p38 and p42/44 mitogen-activated protein kinases and were abrogated by specific inhibitors of both pathways. The impact of TIMP-1 in the anti-invasive action of cisplatin was proven by transfecting cells with small interfering RNA targeting TIMP-1, which completely reversed suppression of invasion by cisplatin. A functional relevance of TIMP-1 upregulation was substantiated by findings showing a concentration-dependent inhibition of Matrigel invasion by recombinant TIMP-1. The essential role of TIMP-1 in the anti-invasive action of cisplatin was confirmed using another human cervical carcinoma cell line (C33A) and human lung carcinoma cells (A549). Altogether, our data demonstrate a hitherto unknown mechanism by which cisplatin exerts its antimetastatic properties on highly invasive cancer cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Biroccio A, Benassi B, Fiorentino F, Zupi G . (2004). Glutathione depletion induced by c-Myc downregulation triggers apoptosis on treatment with alkylating agents. Neoplasia 6: 195–206.
Chan VY, Chan MW, Leung WK, Leung PS, Sung JJ, Chan FK . (2005). Intestinal trefoil factor promotes invasion in non-tumorigenic Rat-2 fibroblast cell. Regul Pept 127: 87–94.
Chintala SK, Ali-Osman F, Mohanam S, Rayford A, Go Y, Gokaslan ZL et al. (1997). Effect of cisplatin and BCNU on MMP-2 levels in human glioblastoma cell lines in vitro. Clin Exp Metastasis 15: 361–367.
Choi J, Choi K, Benveniste EN, Rho SB, Hong YS, Lee JH et al. (2005). Bcl-2 promotes invasion and lung metastasis by inducing matrix metalloproteinase-2. Cancer Res 65: 5554–5560.
Curran S, Murray GI . (2000). Matrix metalloproteinases: molecular aspects of their roles in tumour invasion and metastasis. Eur J Cancer 36: 1621–1630.
Han R, Smith TJ . (2005). Induction by IL-1β of tissue inhibitor of metalloproteinase-1 in human orbital fibroblasts: modulation of gene promoter activity by IL-4 and IFN-γ. J Immunol 174: 3072–3079.
Hartmann JT, Lipp HP . (2003). Toxicity of platinum compounds. Expert Opin Pharmacother 4: 889–901.
Hinz B, Rosch S, Ramer R, Tamm ER, Brune K . (2005). Latanoprost induces matrix metalloproteinase-1 expression in human nonpigmented ciliary epithelial cells through a cyclooxygenase-2-dependent mechanism. FASEB J 19: 1929–1931.
Hong JH, Ahn KS, Bae E, Jeon SS, Choi HY . (2006). The effects of curcumin on the invasiveness of prostate cancer in vitro and in vivo. Prostate Cancer Prostatic Dis 9: 147–152.
Jamieson ER, Lippard SJ . (1999). Structure, recognition, and processing of cisplatin-DNA adducts. Chem Rev 99: 2467–2498.
Khokha R, Waterhouse P, Yagel S, Lala PK, Overall CM, Norton G et al. (1989). Antisense RNA-induced reduction in murine TIMP levels confers oncogenicity on Swiss 3T3 cells. Science 243: 947–950.
Khokha R, Zimmer MJ, Graham CH, Lala PK, Waterhouse P . (1992). Suppression of invasion by inducible expression of tissue inhibitor of metalloproteinase-1 (TIMP-1) in B16-F10 melanoma cells. J Natl Cancer Inst 84: 1017–1022.
Loehrer PJ, Einhorn LH . (1984). Drugs five years later. Cisplatin. Ann Intern Med 100: 704–713.
Mabuchi S, Ohmichi M, Nishio Y, Hayasaka T, Kimura A, Ohta T et al. (2004). Inhibition of NF-κB increases the efficacy of cisplatin in vitro and in vivo ovarian cancer models. J Biol Chem 279: 23477–23485.
Mori K, Shibanuma M, Nose K . (2004). Invasive potential induced under long-term oxidative stress in mammary epithelial cells. Cancer Res 64: 7464–7472.
Morikawa T, Shibuya M, Sakai S, Kudo S . (1995). Effect of anticancer drugs on invasive capacity of human small-cell lung cancer cells in vitro. Nippon Ika Daigaku Zasshi 62: 320–328.
Park MJ, Lee JY, Kwak HJ, Park CM, Lee HC, Woo SH et al. (2005). Arsenic trioxide (As2O3) inhibits invasion of HT1080 human fibrosarcoma cells: role of nuclear factor-κB and reactive oxygen species. J Cell Biochem 95: 955–969.
Razzaque MS, Koji T, Kumatori A, Taguchi T . (1999). Cisplatin-induced apoptosis in human proximal tubular epithelial cells is associated with the activation of the Fas/Fas ligand system. Histochem Cell Biol 111: 359–365.
Stamenkovic I . (2000). Matrix metalloproteinases in tumor invasion and metastasis. Sem Cancer Biol 10: 415–433.
Tong L, Smyth D, Kerr C, Catterall J, Richards CD . (2004). Mitogen-activated protein kinases Erk1/2 and p38 are required for maximal regulation of TIMP-1 by oncostatin M in murine fibroblasts. Cell Signal 16: 1123–1132.
Villedieu M, Deslandes E, Duval M, Heron JF, Gauduchon P, Poulain L . (2006). Acquisition of chemoresistance following discontinuous exposures to cisplatin is associated in ovarian carcinoma cells with progressive alteration of FAK, ERK and p38 activation in response to treatment. Gynecol Oncol 101: 507–519.
Zhang QX, Feng R, Zhang W, Ding Y, Yang JY, Liu GH . (2005). Role of stress-activated MAP kinase p38 in cisplatin- and DTT-induced apoptosis of the esophageal carcinoma cell line Eca 109. World J Gastroenterol 11: 4451–4456.
Acknowledgements
This work was supported by grants from the Deutsche Krebshilfe e.V. (Bonn, Germany), Deutsche Forschungsgemeinschaft (SFB 539, TP BI.6) and Johannes und Frieda Marohn Stiftung (Erlangen, Germany).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramer, R., Eichele, K. & Hinz, B. Upregulation of tissue inhibitor of matrix metalloproteinases-1 confers the anti-invasive action of cisplatin on human cancer cells. Oncogene 26, 5822–5827 (2007). https://doi.org/10.1038/sj.onc.1210358
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210358
Keywords
This article is cited by
-
Synergistic effects of the combination of β-ionone and sorafenib on metastasis of human hepatoma SK-Hep-1 cells
Investigational New Drugs (2012)
-
Cannabinoids, endocannabinoids, and cancer
Cancer and Metastasis Reviews (2011)
-
Decrease of Plasminogen Activator Inhibitor-1 May Contribute to the Anti-Invasive Action of Cannabidiol on Human Lung Cancer Cells
Pharmaceutical Research (2010)