Abstract
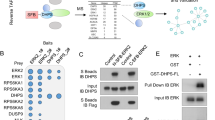
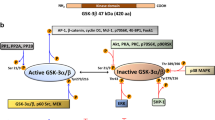
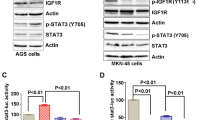
Glycogen-synthase kinase-3 (GSK-3) and extracellular signal-regulated kinase (ERK) are critical downstream signaling proteins for the PI3-kinase/Akt and Ras/Raf/MEK-1 pathway, respectively, and regulate diverse cellular processes including embryonic development, cell differentiation and apoptosis. Here, we show that inhibition of GSK-3 using GSK-3 inhibitors or RNA interference (RNAi) significantly induced the phosphorylation of ERK1/2 in human colon cancer cell lines HT29 and Caco-2. Pretreatment with the PKCδ-selective inhibitor rottlerin or transfection with PKCδ siRNA attenuated the phosphorylation of ERK1/2 induced by the GSK-3 inhibitor SB-216763 and, furthermore, treatment with SB-216763 or transfection with GSK-3α and GSK-3β siRNA increased PKCδ activity, thus identifying a role for PKCδ in the induction of ERK1/2 phosphorylation by GSK-3 inhibition. Treatment with SB-216763 increased expression of cyclooxygenase-2 (COX-2) and IL-8, which are downstream targets of ERK1/2 activation; this induction was abolished by MEK/ERK inhibition, suggesting GSK-3 inhibition induced COX-2 and IL-8 through ERK1/2 activation. The transcriptional induction of COX-2 and IL-8 by GSK-3 inhibition was further demonstrated by the increased COX-2 and IL-8 promoter activity after SB-216763 treatment or transfection with GSK-3α or GSK-3β siRNA. Importantly, our findings identify GSK-3, acting through PKCδ, as a negative regulator of ERK1/2, thus revealing a novel crosstalk mechanism between these critical signaling pathways.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Abbreviations
- GSK-3:
-
glycogen-synthase kinase-3
- COX-2:
-
cyclooxygenase-2
- ERK:
-
extracellular signal-regulated kinase
- LiCl:
-
lithium chloride
- MAPK:
-
mitogen-activated protein kinase
- MBP:
-
myelin basic protein
References
Aikin R, Maysinger D, Rosenberg L . (2004). Endocrinology 145: 4522–4531.
Akhtar M, Watson JL, Nazli A, McKay DM . (2003). FASEB J 17: 1319–1321.
Ali A, Hoeflich KP, Woodgett JR . (2001). Chem Rev 101: 2527–2540.
Bancroft CC, Chen Z, Dong G, Sunwoo JB, Yeh N, Park C et al. (2001). Clin Cancer Res 7: 435–442.
Brew R, Erikson JS, West DC, Kinsella AR, Slavin J, Christmas SE . (2000). Cytokine 12: 78–85.
Brodie C, Blumberg PM . (2003). Apoptosis 8: 19–27.
Chen BC, Chang YS, Kang JC, Hsu MJ, Sheu JR, Chen TL et al. (2004). J Biol Chem 279: 20889–20897.
Choi JH, Hur J, Yoon CH, Kim JH, Lee CS, Youn SW et al. (2004). J Biol Chem 279: 49430–49438.
Claria J . (2003). Curr Pharm Des 9: 2177–2190.
Coghlan MP, Culbert AA, Cross DA, Corcoran SL, Yates JW, Pearce NJ et al. (2000). Chem Biol 7: 793–803.
Corbit KC, Foster DA, Rosner MR . (1999). Mol Cell Biol 19: 4209–4218.
Diehl JA, Cheng M, Roussel MF, Sherr CJ . (1998). Genes Dev 12: 3499–3511.
Ding Q, Wang Q, Evers BM . (2001). Biochem Biophys Res Commun 284: 282–288.
Doble BW, Woodgett JR . (2003). J Cell Sci 116: 1175–1186.
Eickholt BJ, Walsh FS, Doherty P . (2002). J Cell Biol 157: 211–217.
Fang X, Yu S, Tanyi JL, Lu Y, Woodgett JR, Mills GB . (2002). Mol Cell Biol 22: 2099–2110.
Fernando RI, Wimalasena J . (2004). Mol Biol Cell 15: 3266–3284.
Frame S, Cohen P . (2001). Biochem J 359: 1–16.
Ilyas M, Tomlinson IP, Rowan A, Pignatelli M, Bodmer WF . (1997). Proc Natl Acad Sci USA 94: 10330–10334.
Inoue H, Nanayama T, Hara S, Yokoyama C, Tanabe T . (1994). FEBS Lett 350: 51–54.
Jope RS, Johnson GV . (2004). Trends Biochem Sci 29: 95–102.
Kim JW, Lee JE, Kim MJ, Cho EG, Cho SG, Choi EJ . (2003). J Biol Chem 278: 13995–14001.
King WG, Mattaliano MD, Chan TO, Tsichlis PN, Brugge JS . (1997). Mol Cell Biol 17: 4406–4418.
Lee YJ, Soh JW, Dean NM, Cho CK, Kim TH, Lee SJ et al. (2002). Cell Growth Differ 13: 237–246.
Levy L, Neuveut C, Renard CA, Charneau P, Branchereau S, Gauthier F et al. (2002). J Biol Chem 277: 42386–42393.
Li Y, Bharti A, Chen D, Gong J, Kufe D . (1998). Mol Cell Biol 18: 7216–7224.
Mazor M, Kawano Y, Zhu H, Waxman J, Kypta RM . (2004). Oncogene 23: 7882–7892.
Minden A, Karin M . (1997). Biochim Biophys Acta 1333: F85–F104.
Miranti CK, Ohno S, Brugge JS . (1999). J Biol Chem 274: 10571–10581.
Mitsiades CS, Mitsiades N, Poulaki V, Schlossman R, Akiyama M, Chauhan D et al. (2002). Oncogene 21: 5673–5683.
Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B et al. (1997). Science 275: 1787–1790.
Murugappan S, Tuluc F, Dorsam RT, Shankar H, Kunapuli SP . (2004). J Biol Chem 279: 2360–2367.
Pan ZZ, Bruening W, Giasson BI, Lee VM, Godwin AK . (2002). J Biol Chem 277: 35050–35060.
Papadakis KA, Targan SR . (2000). Annu Rev Med 51: 289–298.
Park HS, Kim MS, Huh SH, Park J, Chung J, Kang SS et al. (2002). J Biol Chem 277: 2573–2578.
Perletti GP, Marras E, Concari P, Piccinini F, Tashjian Jr AH . (1999). Oncogene 18: 1251–1256.
Phiel CJ, Klein PS . (2001). Annu Rev Pharmacol Toxicol 41: 789–813.
Plyte SE, Hughes K, Nikolakaki E, Pulverer BJ, Woodgett JR . (1992). Biochim Biophys Acta 1114: 147–162.
Rao R, Hao CM, Breyer MD . (2004). J Biol Chem 279: 3949–3955.
Remacle-Bonnet MM, Garrouste FL, Heller S, Andre F, Marvaldi JL, Pommier GJ . (2000). Cancer Res 60: 2007–2017.
Rommel C, Clarke BA, Zimmermann S, Nunez L, Rossman R, Reid K et al. (1999). Science 286: 1738–1741.
Rossig L, Badorff C, Holzmann Y, Zeiher AM, Dimmeler S . (2002). J Biol Chem 277: 9684–9689.
Schaeffer HJ, Weber MJ . (1999). Mol Cell Biol 19: 2435–2444.
Seger R, Krebs EG . (1995). FASEB J 9: 726–735.
Takada Y, Fang X, Jamaluddin MS, Boyd DD, Aggarwal BB . (2004). J Biol Chem 279: 39541–39554.
Takahashi-Yanaga F, Shiraishi F, Hirata M, Miwa Y, Morimoto S, Sasaguri T . (2004). Biochem Biophys Res Commun 316: 411–415.
Tang Q, Gonzales M, Inoue H, Bowden GT . (2001). Cancer Res 61: 4329–4332.
Tominaga K, Higuchi K, Sasaki E, Suto R, Watanabe T, Fujiwara Y et al. (2004). Aliment Pharmacol Ther 20 (Suppl 1): 143–150.
Tsujio I, Tanaka T, Kudo T, Nishikawa T, Shinozaki K, Grundke-Iqbal I et al. (2000). FEBS Lett 469: 111–117.
Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC et al. (2001). Annu Rev Biochem 70: 535–602.
Wang Q, Ding Q, Dong Z, Ehlers RA, Evers BM . (2000). Anticancer Res 20: 75–83.
Wang Q, Wang X, Evers BM . (2003). J Biol Chem 278: 51091–51099.
Wang Q, Wang X, Hernandez A, Kim S, Evers BM . (2001). Gastroenterology 120: 1381–1392.
Wang Y, Schattenberg JM, Rigoli RM, Storz P, Czaja MJ . (2004). J Biol Chem 279: 31089–31097.
Watcharasit P, Bijur GN, Zmijewski JW, Song L, Zmijewska A, Chen X et al. (2002). Proc Natl Acad Sci USA 99: 7951–7955.
Wilkinson MG, Millar JB . (2000). FASEB J 14: 2147–2157.
Williams C, Shattuck-Brandt RL, DuBois RN . (1999). Ann NY Acad Sci 889: 72–83.
Wong NA, Pignatelli M . (2002). Am J Pathol 160: 389–401.
Zhao D, Keates AC, Kuhnt-Moore S, Moyer MP, Kelly CP, Pothoulakis C . (2001). J Biol Chem 276: 44464–44471.
Zimmermann S, Moelling K . (1999). Science 286: 1741–1744.
Acknowledgements
We thank Eileen Figueroa and Karen Martin for manuscript preparation. This work was supported by Grants RO1 DK48498, R37 AG10885 and PO1 DK35608 from the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Q., Zhou, Y., Wang, X. et al. Glycogen synthase kinase-3 is a negative regulator of extracellular signal-regulated kinase. Oncogene 25, 43–50 (2006). https://doi.org/10.1038/sj.onc.1209004
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209004
Keywords
This article is cited by
-
Unconventional MAPK-GSK-3β Pathway Behind Atypical Epithelial-Mesenchymal Transition In Hepatocellular Carcinoma
Scientific Reports (2017)
-
TSC2/mTORC1 signaling controls Paneth and goblet cell differentiation in the intestinal epithelium
Cell Death & Disease (2015)
-
NFAT5 represses canonical Wnt signaling via inhibition of β -catenin acetylation and participates in regulating intestinal cell differentiation
Cell Death & Disease (2013)
-
Effects of fluoxetine on CRF and CRF1 expression in rats exposed to the learned helplessness paradigm
Psychopharmacology (2013)
-
Mechanisms of RAS/β-catenin interactions
Archives of Toxicology (2013)