Abstract
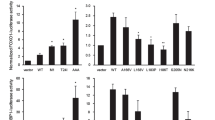
The human c-rel gene (REL), encoding an NF-κB transcription factor, is amplified or mutated in several human B-cell lymphomas and can transform chicken lymphoid cells in vitro. We have previously shown that certain deletions of C-terminal transactivation sequences enhance REL's transforming ability in chicken spleen cells. In this report, we have analysed the effect of single amino-acid changes at select serine residues in the C-terminal transactivation domain on REL's transforming ability. Mutation of either of two TNFα-inducible serine residues (Ser460 and Ser471) to nonphosphorylatable residues (alanine, asparagine, phenylalanine) made REL more efficient at transforming chicken spleen cells in vitro. In contrast, mutation of Ser471 to a phosphorylation mimetic aspartate residue impaired REL's transforming ability, even though it increased REL's inherent transactivation ability as a GAL4-fusion protein. Alanine mutations of several other serine residues within the transactivation domain did not substantially affect REL's transforming ability. Transactivation by GAL4-REL fusion proteins containing either transformation enhancing or nonenhancing mutations at serine residues was generally similar to wild-type GAL4-REL. However, more transforming mutants with mutations at either Ser460 or Ser471 differed from wild-type REL in their ability to transactivate certain κB-site reporter genes. In particular, the SOD2 promoter, encoding manganese superoxide dismutase, was activated less strongly by the more transforming REL mutant REL-S471N in transient assays, but REL-S471N-transformed chicken spleen cells had increased levels of MnSOD protein as compared to wild-type REL-transformed cells. Taken together, our results show that mutations of certain serine residues can enhance REL's transforming ability in vitro and suggest that these mutations increase REL-mediated transformation by altering REL's ability to modulate the expression of select target genes. Furthermore, phosphorylation of Ser471 may be involved in REL-mediated modulation of transformation-specific target gene expression. Lastly, these results suggest that similar mutations in the REL transactivation domain contribute to the development of certain human B-cell lymphomas.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Abbadie C, Kabrun N, Bouali F, Smardova J, Stehelin D, Vandenbunder B and Enrietto PJ . (1993). Cell, 75, 899–912.
Abid MR, Schoots IG, Spokes KC, Wu SQ, Mawhinney C and Aird WC . (2004). J. Biol. Chem., 279, 44030–44038.
Barth TF, Martín-Subero JI, Joos S, Menz CK, Hasel C, Mechtersheimer G, Parwaresch RM, Lichter P, Siebert R and Möller P . (2003). Blood, 101, 3681–3686.
Bash J, Zong W-X and Gélinas C . (1997). Mol. Cell. Biol., 17, 6526–6536.
Bernard D, Monte D, Vandenbunder B and Abbadie C . (2002). Oncogene, 21, 4392–4402.
Bernard D, Quatannens B, Begue A, Vandenbunder B and Abbadie C . (2001). Cancer Res., 61, 2656–2664.
Chen E and Li CC . (1998). Biochem. Biophys. Res. Commun., 249, 728–734.
Epinat J-C, Kazandjian D, Harkness DD, Petros S, Dave J, White DW and Gilmore TD . (2000). Oncogene, 19, 599–607.
Fan Y, Rayet B and Gélinas C . (2004). Oncogene, 23, 1030–1042.
Feuerhake F, Kutok JL, Monti S, Chen W, LaCasce AS, Cattoretti G, Kurtin P, Pinkus GS, de Leval L, Harris NL, Savage KJ, Neuberg D, Habermann TM, Dalla-Favera R, Golub TR, Aster JC and Shipp MA . (2005). Blood, in press.
Ganchi PA, Sun S-C, Greene WC and Ballard DW . (1992). Mol. Cell. Biol., 3, 1339–1352.
Gilmore TD . (1999). Oncogene, 18, 6925–6937.
Gilmore TD, Cormier C, Jean-Jacques J and Gapuzan M-E . (2001). Oncogene, 20, 7098–7103.
Gilmore TD, Kalaitzidis D, Liang M-C and Starczynowski DT . (2004). Oncogene, 23, 2275–2286.
Gilmore TD, White DW, Sarker S and Sif S . (1995). The DNA Provirus: Howard Temin's Scientific Legacy Cooper GM, Greenberg Temin R and Sugden B (eds). American Society for Microbiology Press: Washington, DC, pp 109–128.
Hansen GM, Skapura D and Justice MJ . (2000). Genome Res., 10, 237–243.
Hayden MS and Ghosh S . (2004). Genes Dev., 18, 2195–2224.
Hrdlicková R, Nehyba J and Bose Jr HR . (2001). Mol. Cell. Biol., 21, 6369–6386.
Hrdlicková R, Nehyba J and Humphries EH . (1994). J. Virol., 68, 2371–2382.
Huguet C, Morrice DR, Bouali F, Vandenbunder B, Burt DW and Abbadie C . (1999). Apoptosis, 4, 31–38.
Iwai K, Lee BR, Hashiguchi M, Fukushima A and Iwashima M . (2005). FEBS Lett., 579, 141–144.
Kabrun N, Bumstead N, Hayman MJ and Enrietto PJ . (1989). Mol. Cell. Biol., 10, 4788–4794.
Kalaitzidis D, Davis RE, Rosenwald A, Staudt LM and Gilmore TD . (2002). Oncogene, 21, 8759–8768.
Kalaitzidis D and Gilmore TD . (2002). Genes Chromosomes Cancer, 34, 129–135.
Kralova J, Liss AS, Bargmann W and Bose Jr HR . (1998). Mol. Cell. Biol., 18, 2997–3009.
Kralova J, Liss AS, Bargmann W, Pendleton C, Varadarajan J, Ulug E and Bose Jr HR . (2002). J. Virol., 76, 11960–11970.
Lawrence T, Bebien M, Liu GY, Nizet V and Karin M . (2005). Nature, 434, 1138–1143.
Leung TH, Hoffmann A and Baltimore D . (2004). Cell, 118, 453–464.
Lu D, Thompson JD, Gorski GK, Rice NR, Mayer MG and Yunis JJ . (1991). Oncogene, 6, 1235–1241.
Martin AG and Fresno M . (2000). J. Biol. Chem., 275, 24383–24391.
Martin AG, San-Antonio B and Fresno M . (2001). J. Biol. Chem., 276, 15840–15849.
Mitchell T and Sugden B . (1995). J. Virol., 69, 2968–2976.
Mosialos G, Hamer P, Capobianco AJ, Laursen RA and Gilmore TD . (1991). Mol. Cell. Biol., 11, 5867–5877.
Rayet B, Fan Y and Gélinas C . (2003). Mol. Cell. Biol., 23, 1520–1533.
Sachdev S and Hannink M . (1998). Mol. Cell. Biol., 18, 5445–5456.
Sambrook J, Fritsch EF and Maniatis T . (1989). Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Press: Cold Spring Harbor, NY.
Schatzle JD, Kralova J and Bose Jr HR . (1995). J. Virol., 69, 5383–5390.
Schreiber E, Matthias P, Muller MM and Schaffner W . (1989). Nucleic Acids Res., 17, 6419.
Smith-Sorensen B, Hijmans EM, Beijersbergen RL and Bernards R . (1996). J. Biol. Chem., 271, 5513–5518.
Starczynowski DT, Reynolds JG and Gilmore TD . (2003). Oncogene, 22, 6928–6936.
Wang Y and Gilmore TD . (2001). Biochim. Biophys. Acta, 1538, 260–272.
Yu SH, Chiang WC, Shih HM and Wu KJ . (2004). J. Mol. Med., 82, 621–628.
Acknowledgements
We thank Nancy Rice for anti-REL antisera, Joseph Lipsick for the GAL4-site luciferase reporter plasmid, Manuel Fresno for some of the REL Ser-to-Ala mutants, Ruhul Abid for the SOD2 reporter plasmid, and Henry Bose for the IκBα reporter plasmid. We also thank members of our laboratory for critical reading of the manuscript and helpful discussion. DTS was partially supported by a Pre-doctoral Fellowship from the Natural Sciences & Engineering Research Council of Canada. This work was supported by NIH Grant CA47763 (to TDG).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Starczynowski, D., Reynolds, J. & Gilmore, T. Mutations of tumor necrosis factor α-responsive serine residues within the C-terminal transactivation domain of human transcription factor REL enhance its in vitro transforming ability. Oncogene 24, 7355–7368 (2005). https://doi.org/10.1038/sj.onc.1208902
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1208902