Abstract
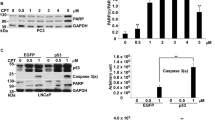
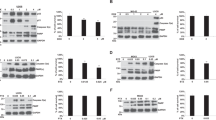
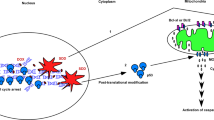
We recently reported that exposure of human cervical carcinoma cells to doxorubicin results in extracellular signal-regulated kinase (ERK)2 activation, which in turn phosphorylates p53 on a previously uncharacterized site, Thr55. This study sought to clarify the biological significance of doxorubicin-induced Thr55 phosphorylation. In breast carcinoma MCF7 cells, doxorubicin (300 nM) activated ERK2 and induced phosphorylation of p53 on Thr55 residues. Pretreatment of MCF7 cells with an ERK2 chemical inhibitor, PD98059 or U0126, blocked doxorubicin-induced p53 activation and suppressed phosphorylation of p53Thr55. MCF55a cells were established by transfection of full-length p53 carrying Thr55 mutation (Thr to Ala) into MCF7 cells. Doxorubicin (500 nM) could not induce p53 activation in MCF55a cells, which showed significantly increased drug resistance toward doxorubicin. While the expression of the apoptotic protein, Bax, showed no difference between MCF7 and MCF55a cells, Bcl-2, an antiapoptotic protein, was constitutively expressed in MCF55a cells. The increase of Bcl-2 protein and/or Bcl-2/Bax ratio might at least partly contribute to the drug resistance of MCF55a cells. In summary, our results suggest that phosphorylation of p53Thr55 by ERK2 is important for doxorubicin-induced p53 activation and cell death.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Appella E and Anderson CW . (2001). Eur. J. Biochem., 268, 2764–2772.
Banin S, Moyal L, Shieh S, Taya Y, Anderson CW, Chessa L, Smorodinsky N, Prives C, Reiss Y, Shiloh Y and Ziv Y . (1998). Science, 281, 1674–1677.
Bates S and Vousden KH . (1996). Curr. Opin. Genet. Dev., 6, 1–7.
Bulavin DV, Saito S, Hollander MC, Sakaguchi K, Anderson CW, Appella E and Fornace Jr AJ . (1999). EMBO J., 18, 6845–6854.
Bushmann T, Potapova O, Ivanov VN, Fuchs SY, Henderson S, Fried VA, Minamoto T, Alarcon-Vargas D, Pincus MR, Gaarde WA, Holbrook NJ, Shiloh Y and Ronai Z . (2001). Mol. Cell. Biol., 21, 2743–2754.
Canman CE, Lim DS, Cimprich KA, Taya Y, Tamai K, Sakaguchi K, Appella E, Kastan MB and Siliciano JD . (1998). Science, 281, 1677–1679.
de Almodóvar CR, Ruiz-Ruiz C, Muňoz-Pinedo C, Robledo G and López-Rivas A . (2001). Oncogene, 20, 7128–7133.
Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery Jr CA, Butel JS and Bradley A . (1992). Nature, 356, 215–221.
Fan S, Smith ML, Rivet DJ, Duba D, Zhan Q, Kohn KW, Fornace Jr AJ and O'Connor PM . (1995). Cancer Res., 55, 1649–1654.
Giaccia A and Kastan MB . (1998). Genes Dev., 12, 2973–2983.
Gottlieb TM and Oren M . (1996). Biochim. Biophys. Acta, 1287, 77–102.
Guillouf C, Rosselli F, Krishnaraju K, Moustacchi E, Hoffman B and Liebermann DA . (1995). Oncogene, 10, 2263–2270.
Haldar S, Negrini M, Monne M, Sabbioni S and Croce CM . (1994). Cancer Res., 54, 2095–2097.
Hayakawa J, Ohmichi M, Kurachi H, Ikegami H, Kimura A, Matsuoka T, Jikihara H, Mercola D and Murata Y . (1999). J. Biol. Chem., 274, 31648.
Hirao A, Kong Y-Y, Matsuoka S, Wakeham A, Ruland J, Yoshida H, Liu D, Elledge SJ and Mak TW . (2000). Science, 287, 1824–1827.
Hockenbery D, Nunez G, Milliman C, Schreiber RD and Korsmeyer SJ . (1990). Nature, 348, 334–336.
Hollstein M, Sidransky D, Vogelstein B and Harris CC . (1991). Science, 253, 49–53.
Huang C, Ma W, Maxiner A, Sun Y and Dong Z . (1999). J. Biol. Chem., 274, 12229–12235.
Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT and Weinberg RA . (1994). Curr. Biol., 4, 1–7.
Johnstone RW, Ruefli AA and Lowe SW . (2002). Cell, 108, 153–164.
Kannan K, Amariglio N, Rechavi G, Jakob-Hirsch J, Kela I, Kaminski N, Getz G, Domany E and Givol D . (2001). Oncogene, 20, 2225–2234.
Knippschild U, Milne DM, Campbell LE, DeMaggio AJ, Christenson E, Hoekstra MF and Meek DW . (1997). Oncogene, 15, 1727–1736.
Ko LJ and Prives C . (1996). Genes Dev., 10, 1054–1072.
Lane DP . (1992). Nature, 358, 15–16.
Lees-Miller SP, Sakaguchi K, Ullrich SJ, Appella E and Anderson CW . (1992). Mol. Cell. Biol., 12, 5041–5049.
Liu L, Scolnick DM, Trievel RC, Zhang HB, Marmorstein R, Halazonetis TD and Berger SL . (1999). Mol. Cell. Biol., 19, 1202–1209.
Marshall CJ . (1998). Cell, 80, 179–185.
Meek DW . (1999). Oncogene, 18, 7666–7675.
Milne DM, Campbell LE, Campbell DG and Meek DW . (1995). J. Biol. Chem., 270, 5511–5518.
Miyashita T, Krajewski S, Krajewska M, Wang HG, Lin HK, Liebermann DA, Hoffman B and Reed JC . (1994). Oncogene, 9, 1799–1805.
Miyashita T and Reed JC . (1995). Cell, 80, 293–299.
Persons DL, Yazlovitskaya EM, Cui W and Pelling JC . (2000a). Clin. Cancer Res., 5, 1007–1014.
Persons DL, Yazlovitskaya EM and Pelling JC . (2000b). J. Biol. Chem., 275, 35778–35785.
Reed JC . (1995). Curr. Opin. Oncol., 7, 541–546.
Rodriguez MS, Desterro JM, Lain S, Midgley CA, Lane DP and Hay RT . (1999). EMBO J., 18, 6455–6461.
Ryan KM, Phillips AC and Vousden KH . (2001). Curr. Opin. Cell Biol., 13, 332–337.
Sax JK and El-Deiry WS . (2003). Cell Death Differ., 10, 413–417.
Sakaguchi K, Herrera JE, Saito S, Miki T, Bustin M, Vassilev A, Anderson CW and Appella E . (1998). Genes Dev., 12, 2831–2841.
Shieh SY, Ahn J, Tamai K, Taya Y and Prives C . (2000). Genes Dev., 14, 289–300.
Shieh SY, Taya Y and Prives C . (1999). EMBO J., 18, 1815–1823.
Tang D, Wu D, Hirao A, Lahti JM, Liu L, Mazza B, Kidd VJ, Mak TW and Ingram AJ . (2002). J. Biol. Chem., 277, 12710–12717.
Tibbetts RS, Brumbaugh KM, Williams JM, Sarkaria JN, Cliby WA, Shieh SY, Taya Y, Prives C and Abraham RT . (1999). Genes Dev., 13, 152–157.
Wang L, Wu Q, Qiu P, Miraz A, McGuirk M, Kirchmeier P, Greene JR, Wang Y, Pickett CB and Liu S . (2001). J. Biol. Chem., 276, 43604–43610.
Wang X, Martinale JL and Holbrook NJ . (2000). J. Biol. Chem., 275, 39435–39443.
Woessmann W, Chen X and Borkhardt A . (2002). Cancer Chemother. Pharmocol., 50, 397–404.
Yeh PY, Chuang SE, Yeh KH, Song YC and Cheng AL . (2001). Biochem. Biophys. Res. Commun., 284, 880–886.
Yeh PY, Chuang SE, Yeh KH, Song YC, Ea CK and Cheng AL . (2002). Biochem. Pharmacol., 63, 1423–1430.
Zhao R, Gish K, Murphy M, Yin Y, Notterman D, Hoffman WH, Tom E, Mack DH and Levine AJ . (2000). Genes Dev., 14, 981–993.
Acknowledgements
This research was supported by grant 89-FA01-1-4 from the Ministry of Education, Taiwan, ROC, and grant 91A1-CANT-1 from the National Health Research Institute, Taiwan, ROC to the Cancer Research Center, College of Medicine, National Taiwan University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yeh, P., Chuang, SE., Yeh, KH. et al. Phosphorylation of p53 on Thr55 by ERK2 is necessary for doxorubicin-induced p53 activation and cell death. Oncogene 23, 3580–3588 (2004). https://doi.org/10.1038/sj.onc.1207426
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1207426
Keywords
This article is cited by
-
Complementary omics strategies to dissect p53 signaling networks under nutrient stress
Cellular and Molecular Life Sciences (2022)
-
LncRNA DANCR represses Doxorubicin-induced apoptosis through stabilizing MALAT1 expression in colorectal cancer cells
Cell Death & Disease (2021)
-
The molecular mechanism of anticancer action of novel octahydropyrazino[2,1-a:5,4-a′]diisoquinoline derivatives in human gastric cancer cells
Investigational New Drugs (2018)
-
Lead induced changes in phosphorylation of PSII proteins in low light grown pea plants
BioMetals (2015)
-
The MEK/ERK pathway is the primary conduit for Borrelia burgdorferi-induced inflammation and P53-mediated apoptosis in oligodendrocytes
Apoptosis (2014)