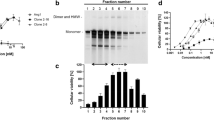
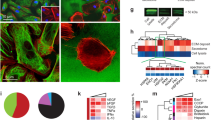
Abstract
The hypothesis that tumor growth is angiogenesis-dependent has been documented by a considerable body of direct and indirect experimental data. Since the discovery of the vascular endothelial growth factor (VEGF), most attention has been focused on the VEGF system. Although fibroblast growth factors 1 and 2 (FGF-1 and FGF-2) can exert a strong angiogenic activity when they are supplied as a single pharmacological agent, their role in pathological angiogenesis in preclinical models remains controversial. To decipher the contribution of FGF receptors in various models of angiogenesis, we took advantage of the anti-idiotypic strategy to obtain circulating agonists specific for FGFR-1 and FGFR-2 (AIdF-1 and AIdF-2). They mimicked FGF-1 and FGF-2 for receptor binding, signal transduction, proliferation of endothelial cells and differentiation of the bladder carcinoma cell NBT-II which expresses FGFR-2b but not FGFR-1. The constitutive expression of FGFR-1 allowed binding of FGF-2 and AIdF-2 and inhibition of the proliferation of NBT-II cells. AIdF-1 and AIdF-2 induced angiogenesis in the corneal pocket assay. Although FGFR-1 dimerization achieved by AIdF-2 injection led to highly differentiated and smaller NBT-II tumors, no sign of reduction of tumor angiogenesis was observed, thus suggesting that endothelial cells are resistant to FGF.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Arnaud E, Touriol C, Boutonnet C, Gensac MC, Vagner S, Prats H and Prats AC . (1999). Mol. Cell. Biol., 19, 505–514.
Basilico C and Moscatelli D . (1992). Adv. Cancer Res., 59, 115–165.
Bikfalvi A, Klein S, Pintucci G and Rifkin DB . (1997). Endocr. Rev., 18, 26–45.
Billottet C, Janji B, Thiery JP and Jouanneau J . (2002). Oncogene, 21, 8128–8139.
Bugler B, Amalric F and Prats H . (1991). Mol. Cell. Biol., 11, 573–577.
Clary DO, Weskamp G, Austin LR and Reichardt LF . (1994). Mol. Biol. Cell, 5, 549–563.
Couraud PO and Strosberg AD . (1991). Biochem. Soc. Trans., 19, 147–151.
Gospodarowicz D . (1987). Int. J. Rad. Appl. Instrum. B, 14, 421–434.
Hutchings H, Maitre-Boube M, Tombran-Tink J and Plouet J . (2002). Biochem. Biophys. Res. Commun., 294, 764–769.
Jerne NK, Roland J and Cazenave PA . (1982). EMBO J., 1, 243–247.
Jonca F, Ortega N, Gleizes PE, Bertrand N and Plouet J . (1997). J. Biol. Chem., 272, 24203–24209.
Jouanneau J, Moens G and Thiery JP . (1999). Oncogene, 18, 327–333.
Jouanneau J, Plouet J, Moens G and Thiery JP . (1997). Oncogene, 14, 671–676.
Kudla AJ, Jones NC, Rosenthal RS, Arthur K, Clase KL and Olwin BB . (1998). J. Cell Biol., 142, 241–250.
Mignatti P, Morimoto T and Rifkin DB . (1991). Proc. Natl. Acad. Sci. USA, 88, 11007–11011.
Miyamoto M, Naruo K, Seko C, Matsumoto S, Kondo T and Kurokawa T . (1993). Mol. Cell. Biol., 13, 4251–4259.
Moscatelli D . (1992). J. Biol. Chem., 267, 25803–25809.
Okada-Ban M, Thiery JP and Jouanneau J . (2000). Int. J. Biochem. Cell. Biol., 32, 263–267.
Ortega N, Jonca F, Vincent S, Favard C, Ruchoux MM and Plouet J . (1997). Am. J. Pathol., 151, 1215–1224.
Ozaki H, Okamoto N, Ortega S, Chang M, Ozaki K, Sadda S, Vinores MA, Derevjanik N, Zack DJ, Basilico C and Campochiaro PA . (1998). Am. J. Pathol., 153, 757–765.
Plouet J and Gospodarowicz D . (1990). Exp. Eye Res., 51, 519–529.
Powers CJ, McLeskey SW and Wellstein A . (2000). Endocr. Relat. Cancer, 7, 165–197.
Quarto N and Amalric F . (1994). J. Cell Sci., 107, 3201–3212.
Rogelj S, Weinberg RA, Fanning P and Klagsbrun M . (1988). Nature, 331, 173–175.
Sato Y and Rifkin DB . (1988). J. Cell Biol., 107, 1199–1205.
Savagner P, Valles AM, Jouanneau J, Yamada KM and Thiery JP . (1994). Mol. Biol. Cell, 5, 851–862.
Schweigerer L, Neufeld G and Gospodarowicz D . (1987). J. Clin. Invest., 80, 1516–1520.
Seghezzi G, Patel S, Ren CJ, Gualandris A, Pintucci G, Robbins ES, Shapiro RL, Galloway AC, Rifkin DB and Mignatti P . (1998). J. Cell Biol., 141, 1659–1673.
Soulet F, Al Saati T, Roga S, Amalric F and Bouche G . (2001). Biochem. Biophys. Res. Commun., 289, 591–596.
Wiedlocha A, Falnes PO, Madshus IH, Sandvig K and Olsnes S . (1994). Cell, 76, 1039–1051.
Acknowledgements
This work was made possible by the financial support of the Ligue Contre le Cancer, the Association pour la Recherche sur le Cancer, the Fondation de France. BM was supported by a fellowship from the Fédération de la Recherche Médicale.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Malavaud, B., Pedron, S., Sordello, S. et al. Direct FGF receptor 1 activation through an anti-idiotypic strategy mimicks the biological activity of FGF-2 and inhibits the progression of the bladder carcinoma derived from NBT-II cells. Oncogene 23, 6769–6778 (2004). https://doi.org/10.1038/sj.onc.1207135
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1207135