Abstract
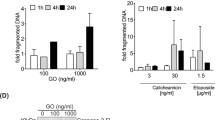
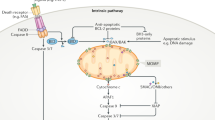
Permeability transition, and a subsequent drop in mitochondrial membrane potential (ΔΨm), have been suggested to be mechanisms by which cytochrome c is released from the mitochondria into the cytosol during apoptosis. Furthermore, a drop in ΔΨm has been suggested to be an obligate early step in the apoptotic pathway. Didemnin B, a branched cyclic peptolide described to have immunosuppressive, anti-tumour, and anti-viral properties, induces rapid apoptosis in a range of mammalian cell lines. Induction of apoptosis by didemnin B in cultured human pro-myeloid HL-60 cells is the fastest and most complete ever described with all cells being apoptotic after 3 h of treatment. By utilizing the system of didemnin B-induced apoptosis in HL-60 cells, and the potent inhibitors of mitochondrial permeability transition, cyclosporin A and bongkrekic acid, we show that permeability transition as determined by changes in ΔΨm and mitochondrial Ca2+ fluxing, is not a requirement for apoptosis or cytochrome c release. In this system, changes in mitochondrial membrane potential and cytochrome c release are shown to be dependent on caspase activation, and to occur concurrently with the release of caspase-9 from mitochondria, genomic DNA fragmentation and apoptotic body formation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
Abbreviations
- AIF:
-
apoptosis inducing factor
- CyA:
-
cyclosporin A
- BA:
-
bongkrekic acid
- JC-1:
-
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide
- PT:
-
permeability transition
- PTP:
-
permeability transition pore
- CCCP:
-
carbonyl cyanide m-chlorophenyl hydrazone
- DAPI:
-
4,6′-diamidino-2-phenylindole
- mtDNA:
-
mitochondrial DNA
- z-VAD-fmk:
-
benzyloxycarbonyl-Val-Ala-DL-Asp-fluoromethylketone
- Ac-DEVD-cho:
-
acetyl-Asp-Glu-Val-L-Asp-aldehyde
- Ac-YVAD-cmk:
-
acetyl-Tyr-Val-Ala-Asp-chloromethylketone
References
Bradford MM . 1976 Anal. Biochem. 72: 248–254
Brenner C, Cadiou H, Vieira HL, Zamzami N, Marzo I, Xie Z, Leber B, Andrews D, Duclohier H, Reed JC, Kroemer G . 2000 Oncogene 19: 329–336
Bossy-Wetzel E, Newmeyer DD, Green DR . 1998 EMBO J. 17: 37–49
Chen Q, Gong B, Almasan A . 2000 Cell. Death Differ. 7: 227–233
Crews CM, Collins JL, Lane WS, Snapper ML, Schreiber SL . 1994 J. Biol. Chem. 269: 15411–15414
Crews CM, Lane WS, Schreiber SL . 1996 Proc. Natl. Acad. Sci. USA 93: 4316–4319
de Bruijn J, Frost DJ, Nugteren DH, Graudemer A, Lijmbach GWM, Cox HC, Berends W . 1973 Tetrahedron 29: 1541–1547
Desagher S, Martinou JC . 2000 Trends Cell. Biol. 10: 369–377
Fournier N, Ducet G, Crevat A . 1987 J. Bioenerg. Biomembr. 19: 297–303
Gamen S, Anel A, Montoya J, Marzo I, Piñeiro A, Naval J . 1995 FEBS Lett. 376: 15–18
Gamen S, Anel A, Perez-Galan P, Lasierra P, Johnson D, Pineiro A, Naval J . 2000 Exp. Cell. Res. 258: 223–235
Goldstein JC, Waterhouse NJ, Juin P, Evan GI, Green DR . 2000 Nat. Cell. Biol. 2: 156–162
Grubb DR, Wolvetang EJ, Lawen A . 1995 Biochem. Biophys. Res. Commun. 215: 1130–1136
Henderson PJ, Lardy HA . 1970 J. Biol. Chem. 245: 1319–1326
Herrmann M, Lorenz H-M, Voll R, Grünke M, Woith W, Kalden JR . 1994 Nucl. Acid. Res. 22: 5506–5507
Ichas F, Mazat JP . 1998 Biochim. Biophys. Acta 1366: 33–50
Jacobson MD, Burne JF, King MP, Miyashita T, Reed JC, Raff MC . 1993 Nature 361: 365–369
Jiang S, Cai J, Wallace DC, Jones DP . 1999 J. Biol. Chem. 274: 29905–29911
Johnson KL, Vaillant F, Lawen A . 1996 FEBS Lett. 383: 1–5
Johnson KL, Grubb DR, Lawen A . 1999 J. Cell. Biochem. 72: 269–278
Johnson KL, Lawen A . 1999 Immunol. Cell. Biol. 77: 242–248
Kim TH, Zhao Y, Barber MJ, Kuharsky DK, Yin XM . 2000 J. Biol. Chem. 275: 39474–39481
Kirsch DG, Doseff A, Chau BN, Lim DS, de Souza-Pinto NC, Hansford R, Kastan MB, Lazebnik YA, Hardwick JM . 1999 J. Biol. Chem. 274: 21155–21161
Klingenberg M, Grebe K, Heldt HW . 1970 Biochem. Biophys. Res. Commun. 39: 344–351
Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD . 1997a Science 275: 1132–1136
Kluck RM, Martin SJ, Hoffman BM, Zhou JS, Green DR, Newmeyer DD . 1997b EMBO J. 16: 4639–4649
Krajewski S, Krajewski M, Ellerby LM, Welsh K, Xie Z, Deveraux QL, Salvesen GS, Bredesen DE, Rosenthal RE, Fiskum G, Reed JC . 1999 Proc. Natl. Acad. Sci. USA 96: 5752–5757
Krohn AJ, Wahlbrink T, Prehn JH . 1999 J. Neurosci. 19: 7394–7404
Larm JA, Vaillant F, Linnane AW, Lawen A . 1994 J. Biol. Chem. 269: 30097–30100
Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X . 1997 Cell 91: 479–489
Lijmbach GWM, Cox HC, Berends W . 1970 Tetrahedron 26: 5993–5999
Liu X, Kim CN, Yang J, Jemmerson R, Wang X . 1996 Cell 86: 147–157
Marchetti P, Castedo M, Susin SA, Zamzami N, Hirsch T, Macho A, Haeffner A, Hirsch F, Geuskens M, Kroemer G . 1996 J. Exp. Med. 184: 1155–1160
Meng L, Sin N, Crews CM . 1998 Biochemistry 37: 10488–10492
Montgomery DW, Zukoski CF . 1985 Transplantation 40: 49–56
Pastorino JG, Chen S-T, Tafani M, Snyder JW, Farber JL . 1998 J. Biol. Chem. 273: 7770–7775
Petronilli V, Cola C, Massari S, Colonna R, Bernardi P . 1993 J. Biol. Chem. 268: 21939–21945
Rinehart Jr KL, Gloer JB, Wilson GR, Hughes Jr RG, Li LH, Renis HE, McGoven JP . 1983 Fed. Proc. 42: 87–90
Robertson JD, Gogvadze V, Zhivotovsky B, Orrenius S . 2000 J. Biol. Chem. 275: 32438–32443
Russel WC, Newman C, Williamson DH . 1975 Nature 253: 4671–462
Rytomaa M, Lehmann K, Downward J . 2000 Oncogene 19: 4461–4468
Saito M, Korsmeyer SJ, Schlesinger PH . 2000 Nat. Cell. Biol. 2: 553–555
Salvioli S, Ardizzoni A, Franceschi C, Cossarizza A . 1997 FEBS Lett. 411: 77–82
Scarlett JL, Murphy MP . 1997 FEBS Lett. 418: 282–286
Scorrano L, Petronilli V, Di Lisa F, Bernardi P . 1999 J. Biol. Chem. 274: 22581–22585
Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, Wang HG, Reed JC, Nicholson DW, Alnemri ES, Green DR, Martin SJ . 1999 J Cell Biol. 144: 281–292
Slee EA, Keogh SA, Martin SJ . 2000 Cell Death Differ. 7: 556–565
Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G . 1999 Nature 397: 441–446
Vaillant F, Nagley P . 1995 Hum. Mol. Genet. 4,: 903–914
Weed SD, Stringfellow DA . 1983 Antiviral Res. 3: 269–274
Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng T-I, Jones DP, Wang X . 1997 Science 275: 1129–1132
Zamzami N, Marchetti P, Castedo M, Decaudin D, Macho A, Hirsch T, Susin SA, Petit PX, Mignotte B, Kroemer G . 1995a J. Exp. Med. 182: 367–377
Zamzami N, Marchetti P, Castedo M, Hirsch T, Susin SA, Masse B, Kroemer G . 1996 FEBS Lett. 384: 53–57
Zamzami N, Marchetti P, Castedo M, Zanin C, Vayssiere JL, Petit PX, Kroemer G . 1995b J. Exp. Med. 181: 1661–1672
Zhai D, Huang X, Han X, Yang F . 2000 FEBS Lett. 472: 293–296
Zhuang J, Dinsdale D, Cohen GM . 1998 Cell Death Differ. 5: 953–962
Zou H, Henzel WJ, Liu X, Lutschg A, Wang X . 1997 Cell 90: 405–413
Acknowledgements
We thank the National Cancer Institute, Drug Synthesis and Chemistry Branch, Bethesda, (USA) for the gift of didemnin B, Prof JA Duine from Delft University of Technology (Netherlands) for the gift of bongkrekic acid, Dr René Traber from Novartis Pharma Ltd (Basel, Switzerland) for the gift of cyclosporin A, Dr Donald Nicholson from Department of Biochemistry and Molecular Biology, Merck Frosst Centre for Therapeutic Research, Quebec, Canada for the gift of anti-caspase-3 antibody. We also thank Mr Barry Veitch for his invaluable assistance with the electron microscopy work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grubb, D., Ly, J., Vaillant, F. et al. Mitochondrial cytochrome c release is caspase-dependent and does not involve mitochondrial permeability transition in didemnin B-induced apoptosis. Oncogene 20, 4085–4094 (2001). https://doi.org/10.1038/sj.onc.1204545
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1204545
Keywords
This article is cited by
-
Natural Products Diversity of Marine Ascidians (Tunicates; Ascidiacea) and Successful Drugs in Clinical Development
Natural Products and Bioprospecting (2017)
-
Drug development from marine natural products
Nature Reviews Drug Discovery (2009)
-
Induction of apoptosis in human prostate cancer cell line, PC3, by 3,3′-diindolylmethane through the mitochondrial pathway
British Journal of Cancer (2004)
-
Antiandrogen-induced cell death in LNCaP human prostate cancer cells
Cell Death & Differentiation (2003)
-
Aplidin™ induces the mitochondrial apoptotic pathway via oxidative stress-mediated JNK and p38 activation and protein kinase C δ
Oncogene (2002)