Abstract
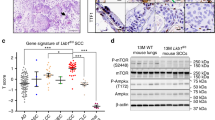
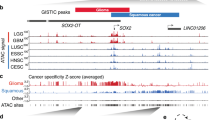
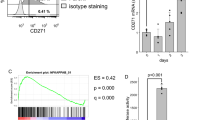
The overexpression of SPRR1B in bronchial epithelium is a marker for early metaplastic changes and the loss of its expression is associated with an irreversible malignant transformation. In the present study, we have used a model system consisting of normal and malignant bronchial epithelial (BE) cells to elucidate the differential transcriptional control of SPRR1B. SPRR1B expression is either detectable or PMA (phorbol 13-myristate 12-acetate) -inducible in several malignant BE cells including squamous, adeno, small and large cell carcinomas. Loss of SPRR1B expression is correlated well with the lack of strong in vivo protein-DNA interactions at the −152 bp promoter, which contains two functional TRE sites. Even though the basal level AP-1 protein DNA binding pattern is different between normal and malignant cells, PMA significantly enhances Jun and Fos binding to the consensus TRE site in both cell types. Intriguingly, the composition of AP-1 protein binding to the −152 to −86 bp SPRR1B promoter is quite different. In untreated cells, SPRR1B promoter is predominantly occupied by JunD and Fra2. PMA significantly induced binding of JunB and Fra1 in normal cells, while JunB and Fra2 bound to TREs in the malignant cells. Overexpression of fra1 in malignant cells significantly enhanced SPRR1B promoter activity. In contrast, overexpression of fra2, but not fra1, strongly reduced both basal and PMA-inducible promoter activities in normal cells. Together, these results indicate that either temporal expression and/or differential activation of AP-1 proteins, especially Fra1 and Fra2, might contribute to the dysregulation of terminal differentiation marker, SPRR1B, expression in various BE cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Abate C, Baker SJ, Lees-Miller SP, Anderson CW, Marshak DR and Curran T. . 1993 Proc. Natl. Acad. Sci. USA 90: 6766–6770.
Abraham JM, Wang S, Suzuki H, Jiang HY, Rosenblum-Vos LS, Yin J and Meltzer SJ. . 1996 Cell Growth Differ. 7: 855–860.
Anisowicz A, Sotiropoulou G and Sager R. . 1999 Mol. Med. 5: 526–541.
Basbaum C and Jany B. . 1990 Am. J. Physiol. 259: L38–L46.
Carrasco D and Bravo R. . 1995 Oncogene 10: 1069–1079.
DeMuth JP, Weaver DA, Crawford EL, Jackson CM and Willey JC. . 1998 Am. J. Respir. Cell. Mol. Biol. 19: 25–29.
Deng J, Pan R and Wu R. . 2000 J. Biol. Chem. 275: 5739–5747.
DiSepio D, Bickenbach JR, Longley MA, Bundman DS, Rothnagel JA and Roop DR. . 1999 Differentiation 64: 225–235.
Eckert RL and Welter JF. . 1996 Mol. Biol. Rep. 23: 59–70.
Fischer DF, Gibbs S, van De Putte P and Backendorf C. . 1996 Mol. Cell. Biol. 16: 5365–5374.
Fischer DF, Sark MW, Lehtola MM, Gibbs S, van de Putte P and Backendorf C. . 1999 Genomics 55: 88–99.
Fong KM, Sekido Y and Minna JD. . 1999 J. Thorac. Cardiovasc. Surg. 118: 1136–1152.
Fujimoto W, Marvin KW, George MD, Celli G, Darwiche N, De Luca LM and Jetten AM. . 1993 J. Invest. Dermatol. 101: 268–274.
Gruda MC, Kovary K, Metz R and Bravo R. . 1994 Oncogene 9: 2537–2547.
Hu R, Wu R, Deng J and Lau D. . 1998 Lung Cancer 20: 25–30.
Jetten AM. . 1989 Environ. Health Perspect. 80: 149–160.
Jetten AM, Nervi C and Vollberg TM. . 1992 J. Natl. Cancer Inst. Monogr. 13: 93–100.
Karin M, Liu Z and Zandi E. . 1997 Curr. Opin. Cell. Biol. 9: 240–246.
Lau D, Xue L, Hu R, Liaw T, Wu R and Reddy S. . 2000 Am. J. Respir. Cell. Mol. Biol. 22: 92–96.
Lee HY, Chaudhary J, Walsh GL, Hong WK and Kurie JM. . 1998 Oncogene 16: 3039–3046.
Lohman FP, Medema JK, Gibbs S, Ponec M, van de Putte P and Backendorf C. . 1997 Exp. Cell. Res. 231: 141–148.
Marks PA, Richon VM and Rifkind RA. . 2000 J. Natl. Cancer Inst. 92: 1210–1216.
McClaren M and Isseroff RR. . 1994 J. Invest. Dermatol. 102: 375–381.
Momparler RL and Bovenzi V. . 2000 J. Cell. Physiol. 183: 145–154.
Mueller PR and Wold B. . 1989 Science 246: 780–786.
Murakami M, Sonobe MH, Ui M, Kabuyama Y, Watanabe H, Wada T, Handa H and Iba H. . 1997 Oncogene 14: 2435–2444.
Pfeifer AM, Lechner JF, Masui T, Reddel RR, Mark GE and Harris CC. . 1989 Environ. Health Perspect. 80: 209–220.
Reddy SP, Chuu YJ, Lao PN, Donn J, Ann DK and Wu R. . 1995 J. Biol. Chem. 270: 26451–26459.
Rutberg SE, Saez E, Lo S, Jang SI, Markova N, Spiegelman BM and Yuspa SH. . 1997 Oncogene 15: 1337–1346.
Sark MW, Fischer DF, de Meijer E, van de Putte P and Backendorf C. . 1998 J. Biol. Chem. 273: 24683–24692.
Schreiber E, Matthias P, Muller MM and Schaffner W. . 1989 Nucleic. Acids Res. 17: 6419.
Soto U, Denk C, Finzer P, Hutter KJ, zur Hausen H and Rosl F. . 2000 Int. J. Cancer 86: 811–817.
Steinert PM, Candi E, Kartasova T and Marekov L. . 1998 J. Struct. Biol. 122: 76–85.
Suzuki, T, Okuno H, Yoshida T, Endo T, Nishina H and Iba H. . 1991 Nucleic. Acids Res. 19: 5537–5542.
Tesfaigzi J and Carlson DM. . 1999 Cell. Biochem. Biophys. 30: 243–265.
Vuong H, Patterson T, Shapiro P, Kalvakolanu DV, Wu R, Ma WY, Dong Z, Kleeberger SR and Reddy SP. . 2000 J. Biol. Chem. 275: 32250–32259.
Welter JF, Crish JF, Agarwal C and Eckert RL. . 1995 J. Biol. Chem. 270: 12614–12622.
Yar M, Eller MS, Bhawan J, Harkness DD, DiBenedetto PJ and Gilchrest BA. . 1995 Exp. Cell. Res. 217: 217–226.
Yuspa SH, Dlugosz AA, Denning MF and Glick AB. . 1996 J. Investig. Dermatol. Symp. Proc. 1: 147–150.
Acknowledgements
The authors would like to thank Drs M Birrer, E Tulchinsky and D Cohen for providing us with the pCMV expression vectors of c-Jun, fra1 and fra2, respectively. We also thank Dr R Isseroff for providing us with primary cultured human epidermal keratinocytes. This work was supported in parts by grants from NIH HL-58122 (to SPM Reddy), ES-06230 (to R Wu), CA-71401 and CA-78282 (to DV Kalvakolanu). We would like to acknowledge the Johns Hopkins Urban Environmental Center (supported by grant from NIEHS ES03819) for use of its core facilities.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Patterson, T., Vuong, H., Liaw, YS. et al. Mechanism of repression of squamous differentiation marker, SPRR1B, in malignant bronchial epithelial cells: role of critical TRE-sites and its transacting factors. Oncogene 20, 634–644 (2001). https://doi.org/10.1038/sj.onc.1204134
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1204134
Keywords
This article is cited by
-
Single-cell RNA-sequencing reveals radiochemotherapy-induced innate immune activation and MHC-II upregulation in cervical cancer
Signal Transduction and Targeted Therapy (2023)
-
Integrative analysis of non-small cell lung cancer patient-derived xenografts identifies distinct proteotypes associated with patient outcomes
Nature Communications (2022)
-
Identification of oral squamous cell carcinoma markers MUC2 and SPRR1B downstream of TANGO
Journal of Cancer Research and Clinical Oncology (2021)
-
Mitogen regulated induction of FRA-1 proto-oncogene is controlled by the transcription factors binding to both serum and TPA response elements
Oncogene (2005)
-
Gene expression-based, individualized outcome prediction for surgically treated lung cancer patients
Oncogene (2004)