Abstract
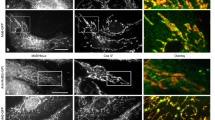
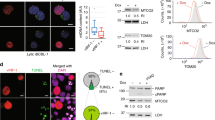
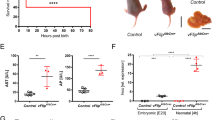
Hepatitis B virus (HBV) X protein activates many viral and cellular genes in trans and functional disruption of the p53 tumor suppressor gene product occurs when X protein is transiently expressed in the cytoplasm of cultured cells. We have carried out investigations to determine the exact location of X protein in X gene transfected cells by using a fluorescent staining technique as well as by biochemical analyses. Aggregation of mitochondrial structures became evident at the periphery of nucleus in the cytoplasm of X transfected cells. X protein was found associated with the aggregated mitochondrial structures. Furthermore, transiently expressed p53 protein co-localized with X protein in X transfected cells. However, the appearance of aggregated mitochondrial structures at the nuclear periphery was independent of the presence of p53 protein in X transfected cells. X protein expression also caused an appearance of TUNEL positive nucleus, cytochrome c release from mitochondrial, the decrease of mitochondrial membrane potential and the membrane blebbing of X transfected cells, which are characteristic of cell death. Our data suggest that X protein causes an abnormal aggregation of mitochondrial structures in the cell, which may be eventually connected with cell death.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Arii M, Takada S and Koike K. . 1992 Oncogene 7: 397–403.
Benn J and Schneider RT. . 1994 Proc. Natl. Acad. Sci. USA 91: 10350–10354.
Cross JC, Wen P and Rutter WJ. . 1993 Proc. Natl. Acad. Sci. USA 90: 8078–8082.
Ganem D and Varmus HE. . 1987 Annu. Rev. Biochem. 56: 651–695.
Hohne M, Schaefer S, Scifer M, Feitelson MA, Paul D and Gerlich WH. . 1990 EMBO J. 9: 1137–1145.
Kekule AS, Lauer U, Weiss L, Luber B and Hofschneider PH. . 1993 Nature 361: 742–745.
Kim C-M, Koike K, Saito I, Miyamura T and Jay G. . 1991 Nature 351: 317–320.
Kim DW, Uetsuki T, Kaziro K, Yamaguchi N and Sugano S. . 1990 Gene 91: 217–223.
Maguire HF, Hoeffler JP and Siddiqui A. . 1991 Science 252: 842–844.
Masuda H, Miller C, Koeffler HP, Battifora H and Cline MJ. . 1987 Proc. Nat. Acad. Sci. USA 84: 7716–7719.
Natoli G, Avantaggiati ML, Chirillo P, Costanzo A, Artini M, Balsano C and Levrero M. . 1994a Mol. Cell. Biol. 14: 989–998.
Natoli G, Avantaggiati ML, Chirillo P, Puri P, Ianni A, Balsano C and Levrero M. . 1994b Oncogene 9: 2837–2843.
Petit PX, Susin S-A, Zamzami N, Mignotte B and Kroemer G. . 1996 FEBS Lett. 396: 7–13.
Rakotomahanina CK, Hilger C, Fink T, Zentgraf H and Schroder CH. . 1994 Oncogene 9: 2613–2621.
Reed JC. . 1997 Nature 387: 773–776.
Rossner MT. . 1992 J. Med. Virol. 36: 101–117.
Sambrook J, Fritsch EF and Maniatis T. . (1989) In: Molecular Cloning: A laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, New York.
Shaulsky G, Goldfinger N, Tosky MS, Levine AJ and Rotter V. . 1991 Oncogene 6: 2055–2065.
Shirakata Y, Kawada M, Fujiki Y, Sano H, Oda M, Yaginuma K, Kobayashi M and Koike K. . 1989 Jpn. J. Cancer Res. 80: 617–621.
Spandau DF and Lee C-H. . 1988 J. Virol. 62: 427–434.
Takada S, Kaneniwa N, Tsuchida N and Koike K. . 1997 Oncogene 15: 1895–1901.
Takada S, Kido H, Fukutomi A, Mori T and Koike K. . 1994 Oncogene 9: 341–348.
Takada S and Koike K. . 1990 Jpn. J. Cancer Res. 81: 1191–1194.
Takada S and Koike K. . 1994 Virology 205: 503–510.
Tanaka S and Koike K. . 1978 Biochim. Biophys. Res. Comm. 81: 791–797.
Tanaka Y, Kanai Y, Okada Y, Nonaka S, Takeda S, Harada A and Hirokawa N. . 1998 Cell 93: 1147–1158.
Terradillos O, Pollicino T, Lecoeur H, Tripodi M, Gougeon M-L, Tiollais P and Buendia MA. . 1998 Oncogene 17: 2115–2123.
Tiollais P, Pourcel C and Dejean A. . 1985 Nature 317: 489–495.
Vander Heiden MG, Chandel NS, Williamson EK, Schumacker PT and Thompson CB. . 1997 Cell 91: 627–637.
Yaffe MP. . 1999 Science 283: 1493–1497.
Acknowledgements
We thank VP Iyemere for reading the manuscript and M Kobayashi for preparing the manuscript. This work was supported partly by a Grant-in-Aid from the Ministry of Education, Science, Sports and Culture and a Grant-in-Aid from the Ministry of Health and Welfare, Japan to K Koike. The work was also supported partly by a grant from Mitsui Life Social Welfare Foundation, Tokyo, Japan to K Koike.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Takada, S., Shirakata, Y., Kaneniwa, N. et al. Association of hepatitis B virus X protein with mitochondria causes mitochondrial aggregation at the nuclear periphery, leading to cell death. Oncogene 18, 6965–6973 (1999). https://doi.org/10.1038/sj.onc.1203188
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1203188
Keywords
This article is cited by
-
Mitochondria ubiquitin ligase, MARCH5 resolves hepatitis B virus X protein aggregates in the liver pathogenesis
Cell Death & Disease (2019)
-
HBV infection increases the risk of macular degeneration: the roles of HBx-mediated sensitization of retinal pigment epithelial cells to UV and blue light irradiation
Journal of Translational Medicine (2018)
-
Thyroid hormone protects hepatocytes from HBx-induced carcinogenesis by enhancing mitochondrial turnover
Oncogene (2017)
-
Hepatitis B virus X protein in liver tumor microenvironment
Tumor Biology (2016)
-
GSIV serine/threonine kinase can induce apoptotic cell death via p53 and pro-apoptotic gene Bax upregulation in fish cells
Apoptosis (2016)