Abstract
Aim:
To test the hypothesis that neonatal hyperoxia induced pulmonary hypertension accompanied by increased Rho-kinase expression in rat lungs and that Rho-kinase inhibitor could attenuate right ventricular hypertrophy and pulmonary arterial remodeling.
Methods:
Newborn rats were exposed to >95% O2 in the first week after birth, then to 60% O2 in the following 2 weeks. Control pups were exposed to room air over the same periods. The pups were injected with either Rho-kinase inhibitor Y-27632 (10 mg·kg−1·d−1, ip) or vehicle from postnatal d 14 to 20. Lung and heart tissues were collected on postnatal d 7 and 21. Rho-kinase activity in lungs was measured using Western blotting and immunohistochemistry. The right ventricular hypertrophy and arterial medial wall thickness (MWT) were assessed morphologically.
Results:
Rho-kinase activity in lungs was comparable between the hyperoxic and control pups on postnatal d 7, but it had a more than 2-fold increase in the hyperoxic pups on postnatal d 21. Moreover, the hyperoxic exposure induced structural features of pulmonary hypertension, as shown by the right ventricular hypertrophy and significantly increased arterial MWT. Administration with Y-27632 effectively blocked the hyperoxia-induced increase of Rho-kinase activity in lungs, and attenuated the right ventricular hypertrophy.
Conclusion:
Rho-kinase inhibitor may be a novel therapy for attenuating the hyperoxia-induced structural changes in pulmonary hypertension.
Similar content being viewed by others
Introduction
Oxygen therapy is an important component in the management of various conditions of respiratory distress in neonates. However, prolonged exposure to hyperoxia in neonates can cause hyperoxic lung injury. Neonatal mice exposed to hyperoxia showed diffuse alveolar damage, increased terminal air space size, and increased lung fibrosis that was similar to human bronchopulmonary dysplasia1,2. Rat pups exposed to high oxygen levels during the alveolar period exhibit decreased alveolarization and pulmonary hypertension, as evidenced by increased muscularization of the peripheral arteries and medial hypertrophy of the muscular arteries3,4,5,6,7.
The small GTP-binding protein Rho and its downstream effector Rho-kinase play an important role in regulating vascular smooth muscle tone8. The Rho-kinase system is constitutively active in regulating vasoconstrictor tone, and upregulation of this pathway occurs in various cardiovascular diseases9,10. Rho-kinase is found to be upregulated in animal models of pulmonary hypertension, and Rho-kinase inhibitors decrease the pulmonary arterial pressure in rodents with monocrotaline and chronic hypoxia-induced pulmonary hypertension11,12,13. Rho-kinase has been identified as a potential therapeutic target in pulmonary hypertension14. However, the therapeutic effect of Rho-kinase inhibitor on hyperoxia-induced pulmonary hypertension is unknown. This study tested the hypotheses that neonatal hyperoxia induces pulmonary hypertension and is accompanied by an increase in Rho-kinase expression and that Rho-kinase inhibitor may attenuate heart structural changes in rats.
Materials and methods
Animals and exposure to hyperoxia
The study was performed in accordance with guidelines provided and approved by the Animal Care and Use Committee of Taipei Medical University (Taiwan, China). Time-dated pregnant Sprague-Dawley rats were housed in individual cages. Within 12 h of birth, litters were pooled and randomly redistributed to the newly delivered mothers, and then exposed to either hyperoxia (experimental group) or room air (control group). Nursing mothers were rotated between the experimental and control litters every 24 h to avoid oxygen toxicity in the mothers. The control groups were kept in normoxia for 1 and 3 weeks. The hyperoxia groups were exposed to >95% O2 for 1 week and were then placed in an environment with 60% O2 for a further 2 weeks. Oxygen exposure was carried out in a modified controller for the basic model (NexBiOxy, Hsinchu, Taiwan, China). Rat pups were injected intraperitoneally with either Rho-kinase inhibitor Y-27632 (Sigma-Aldrich, St Louis, MO, USA) at a dose of 10 mg·kg−1·d−1 or an equivalent volume of vehicle (normal saline) from postnatal d 14 to 20 (Figure 1). The dose and duration of Y-27632 were based on Ziino et al and Xu et al studies15,16. Animals were killed by an intraperitoneal injection of pentobarbital sodium and exsanguinated by aortic transaction on postnatal d 7 and 21. Lung and heart from room air- and hyperoxia-exposed pups were harvested on postnatal d 7 and 21. Body and heart and lung weights were recorded at the time of sacrifice.
Western blotting analysis for ERM family
To quantify Rho-kinase activity, we performed Western blotting analysis for the total and phosphorylated ERM (ezrin, radixin, and moesin) family, a substrate of Rho-kinase (n=4 samples per group). Activated Rho-kinase has been shown to directly phosphorylate COOH-terminal threonine residues of the ERM proteins to regulate their function, and relative Rho-kinase activity is determined as a measure of the ratio of phosphorylated to total ERM11. The primary antibodies used in this study were goat anti-p-ERM (1:200, Santa Cruz Biotechnology Inc, Santa Cruz, CA, USA), goat anti-ERM (Santa Cruz, 1:200), and mouse anti-β-actin (Sigma-Aldrich, 1:200 000). After incubation with the primary antibody, the membranes were probed with the appropriate horseradish peroxidase-conjugated secondary antibody (anti-mouse or anti-goat, 1:20 000; Pierce, Rockford, IL, USA). Immune complexes were visualized using enhanced chemiluminescence detection reagents (Pierce). Densitometric analysis was performed to measure the intensity of Western blotting bands using AIDA software (Advanced Image Data Analyzer; Raytest Izotopenmessgeraete, Straubenhardt, Germany).
Immunohistochemistry
Rho activation was evaluated by analysis of site-specific phosphorylation of Rho-kinase substrate myosin-binding subunit of myosin-associated phosphatase type 1 (MYPT1). Slides were incubated with goat polyclonal anti-p-MYPT1 (Thr 696) antibodies (1:100; Santa Cruz) as the primary antibody. Proliferation of pulmonary artery smooth muscle cells was assessed using anti-proliferating cell nuclear antigen (PCNA) rabbit monoclonal antibody (1:100; Epitomics, Burlingame, CA, USA). The sections were then treated with biotinylated rabbit anti-goat IgG (1:200, Jackson ImmunoResesarch Labotories Inc, PA, USA). Thereafter it was reacted with reagents from an ABC kit (Avidin-Biotin Complex, Vector, CA, USA) according to the manufacturer's recommendations. The optical density values of p-MYPT1 were measured using Image pro plus software version 6.0 (Media Cybernetics, USA) under 200×objective lens of each slice for semiquantitative analysis17. Proliferation was assessed by examining arteries (20 to 60 μm in diameter) in 4 randomly chosen fields in each section and expressed as a percentage of the total number of arteries examined18.
Right ventricular hypertrophy and pulmonary arterial remodeling
The hearts were removed and placed in fixative for 48 h at room temperature. After serial dehydration in alcohol, the tissues were embedded in paraffin. The 7 μm lung sections were stained with hematoxylin and eosin for light microscopy and morphometric analysis. Right ventricular hypertrophy (RVH) was assessed by measuring the thickness ratio of right ventricle (RV)/[left ventricle (LV)+interventricular septum (IVS)]8. The medial wall thickness (MWT) was measured in vessels with an external diameter of 20 to 65 μm. Wall thickness and external diameter were measured under Image-Pro Plus. Fourteen to twenty-six vessels were measured in each animal. The percent medial thickness of an individual vessel was calculated by the following formula: (medial thickness×2×100)/external diameter19.
Statistical analysis
Data are expressed as the mean±SD. Comparisons between the room air and experimental groups were made using Student's t-test on postnatal d 7 and one-way ANOVA with post-hoc Tukey's test on postnatal d 21. Differences were considered significant at P<0.05.
Results
Three pregnant dams were assigned to both room air and hyperoxia groups for 1 week and four pregnant dams were assigned to Rho-kinase inhibition experiment on postnatal d 21. There was no mortality in the rat pups.
Body weight, organ weight, and organ/body weight ratio (%) in rats
All rat pups survived during the study period. The effects of hyperoxia on the rats' body weight, organ weight, and organ/body weight ratio (%) are shown in Table 1. Rats exposed to hyperoxia exhibited significantly lower body and organ weights compared with room-air controls on postnatal d 7 and 21. Heart/body weight and lung/body weight ratios were significantly lower in the hyperoxia-exposed rats on postnatal d 7, relative to the controls. However, on postnatal d 21, although the hyperoxia-exposed rats still showed significantly lower body and organ weights compared with room air-exposed rats, the difference in heart/body weight ratio between the hyperoxia-exposed and control groups was not statistically significant.
Rho-kinase activity
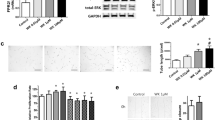
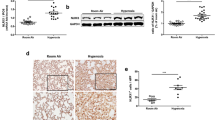
Lungs from the control and experimental groups showed similar levels of Rho-kinase activity on postnatal d 7, as indicated by the ratio of phosphorylated ERM to ERM (ezrin, radixin, and moesin) (Figure 2A). In the group exposed to hyperoxia, this ratio was significantly increased by postnatal d 21, indicating increased activation of Rho-kinase (Figure 2B). Rho-kinase inhibitor treatment for 1 week reduced the hyperoxia-induced increase of Rho-kinase activity. We also investigated the effects of hyperoxia on Rho-kinase activity in lung tissues by conducting immunohistochemical analysis of p-MYPT1. The immunoreactivity was localized in the smooth muscle cells of the vessels and was enhanced in the lung tissues of hyperoxia-exposed rats on postnatal d 21 (Figure 3A). Decreased p-MYPT1 immunoreactivity was observed in the control rats. Rho-kinase inhibitor treatment for 1 week decreased the hyperoxia-induced increase of p-MYPT1 immunoreactivity (Figure 3B).
Lung Rho-kinase activity in (A) 7-d-old rats exposed to room air or hyperoxia and (B) 21-day-old rats exposed to room air or hyperoxia from birth and received Y-27632 or saline from postnatal d 14 to 20. Representative Western blotting for phosphorylated (p) ERM proteins and total ERM and scanning densitometry results for quantification of the protein. Rho-kinase activities were comparable between room air- and hyperoxia-exposed rats on postnatal d 7. Hyperoxia+saline group exhibited a significantly higher Rho-kinase activity compared with room air-exposed groups and hyperoxia+Y-27632 groups on postnatal d 21 (n=4 samples per group. bP<0.05).
Representative photomicrographs and optical density of p-MYPT1. (A) The ROCK activity was investigated in the rat lung sections by immunostain for p-MYPT1, as described in the Methods section. Positive immunoreactivity is indicated by brown staining. The immunoreactivity was localized in the smooth muscle cells of the vessels (v; arrow) and was enhanced in the lung tissues of hyperoxia-exposed rats on postnatal d 21. Rho-kinase inhibitor treatment for 1 week decreased the hyperoxia-induced increase of MYPT1 immunoreactivity. (B) Optical densities of p-MYPT1 were comparable between room air- and hyperoxia-exposed rats on postnatal d 7. Rats exposed to hyperoxia+saline displayed significantly higher optical density of p-MYPT1 compared with room air-exposed groups on postnatal d 21 (cP<0.01). Rho-kinase inhibitor treatment decreased the hyperoxia-induced increase of p-MYPT1 (cP<0.01 vs hyperoxia+saline group).
Right ventricular hypertrophy and pulmonary arterial remodeling
As an indicator of RVH, we calculated the thickness ratio of RV/(LV+IVS). We also estimated arterial remodeling by measuring the MWT of the small pulmonary arteries because MWT is a surrogate marker of pulmonary hypertension20. RV/(LV+IVS) ratios and MWT were comparable between room air- and hyperoxia-exposed rats on postnatal d 7 (Figures 4A and 5A). Chronic exposure to hyperoxia was associated with a significant increase in RVH and MWT by 30% and 10% compared to the room air-exposed controls on postnatal d 21, respectively (Figures 4B and 5B). Rho-kinase inhibitor treatment for 1 week (hyperoxia+Y-27632 group) tended to decrease the hyperoxia-induced increase of RVH compared to hyperoxia+saline group on postnatal d 21 (P=0.064).
RV/(LV+IVS) ratios in 7-d-old rats exposed to room air or hyperoxia and 21-d-old rats exposed to room air or hyperoxia from birth and received Y-27632 or saline from postnatal d 14 to 20. (A) RV/LV+IVS ratios were comparable between room air- and hyperoxia-exposed rats on postnatal d 7. (B) Rats exposed to hyperoxia+saline displayed significantly higher RVH, as shown by an increased RV/LV+IVS ratio compared with room air+saline group on postnatal d 21 (bP<0.05). Rho-kinase inhibitor treatment for 1 week (hyperoxia+Y-27632 group) tended to decrease the hyperoxia-induced increase of RVH (P=0.064 vs hyperoxia+saline group).
MWT in 7-d-old rats exposed to room air or hyperoxia and 21-d-old rats exposed to room air or hyperoxia from birth and received Y-27632 or saline from postnatal d 14 to 20. (A) MWT values were comparable between room air- and hyperoxia-exposed rats on postnatal d 7. (B) Rats exposed to hyperoxia+saline displayed a significantly higher MWT compared with room air+saline group on postnatal d 21 (bP<0.05).
Pulmonary artery cell proliferation
Representative PCNA staining and quantification of the percentage of PCNA-positive pulmonary arteries are presented in Figure 6. The number of PCNA-positive pulmonary arteries was comparable between room air- and hyperoxia-exposed rats on postnatal d 7. Rats exposed to hyperoxia+saline had significantly higher numbers of PCNA-positive pulmonary arteries compared with room air-exposed groups on postnatal d 21. Rho-kinase inhibitor treatment decreased the hyperoxia-induced increase of PCNA-positive pulmonary arteries.
(A) Representative PCNA staining and (B) quantification of the percentage of PCNA-positive pulmonary arteries in 7-d-old rats exposed to room air or hyperoxia and 21-d-old rats exposed to room air or hyperoxia from birth and received Y-27632 or saline from postnatal d 14 to 20. PCNA positive cells were found in the smooth muscle layer of small pulmonary artery (a, arrow). The numbers of PCNA-positive pulmonary arteries were comparable between room air- and hyperoxia-exposed rats on postnatal d 7. Rats exposed to hyperoxia+saline had significantly higher numbers of PCNA-positive pulmonary arteries compared with room air-exposed groups on postnatal d 21 (cP<0.01). Rho-kinase inhibitor treatment decreased the hyperoxia-induced increase of PCNA-positive pulmonary arteries (cP<0.01 vs hyperoxia+saline group). PCNA, proliferating cell nuclear antigen.
Discussion
Our in vivo model showed that exposure of neonatal rats to hyperoxia and further prolonged exposure to a lower concentration of oxygen induced pulmonary hypertension, as shown by increased RVH and arterial MWT. These phenomena were associated with increased p-ERM expression, and Rho-kinase inhibitor treatment reduced Rho-kinase activity and tended to decrease the hyperoxia-induced increase in RVH in the third postnatal week. These results suggested that Rho-kinase inhibitor may provide a novel therapy in attenuating hyperoxia-induced structural changes in pulmonary hypertension.
Murine alveolar development begins on postnatal d 4 and saccular division is completed by the fourteenth day21. Newborn rats are appropriate for the study of neonatal oxygen injury because the developmental stage of the rodent lung at birth parallels that of the human preterm neonate at 24 to 28 weeks of gestation3. During the first 3 weeks of this study, the body weight of experimental rats was approximately 85% and 60% that of the controls on postnatal d 7 and 21, respectively; lung and heart weights were affected similarly to body weight. The hyperoxia-exposed rats exhibited significantly lower body and organ weights and organ/body weight ratios compared with room-air controls on postnatal d 7. However, on postnatal d 21, although the hyperoxia-exposed rats still showed significantly lower body and organ weights, the difference in heart/body weight ratio between the hyperoxia-exposed and control groups was not statistically significant. Rho-kinase inhibitor Y-27632 treatment for 1 week does not influence body weight, organ weight, or organ/body weight ratio in room air- or hyperoxia-exposed rats on postnatal d 21.
The small GTPase RhoA (a member of the Rho family of small GTP-binding proteins) and its downstream effector Rho-kinase play a major role in regulating various cellular functions22,23. Rho/Rho kinase-mediated Ca2+ sensitization is a key component in the sustained vasoconstriction induced by G protein-coupled receptor agonists. Y-27632 is the best-characterized Rho kinase inhibitor that selectively targets P160-Rho kinase from the family of Rho-associated protein kinases24. Although Y-27632 has been shown to attenuate the hypoxic pulmonary vasoconstrictor response in rodents, the effects of Y-27632 on pulmonary arterial remodeling induced by hyperoxia have not been examined in the intact rats25. Enomoto et al found that short-term hyperoxia exposure immediately after birth increased Rho-kinase activity in male newborn rat lungs26. In this study, we measured whole lung Rho-kinase activity in both sexes and found that Rho-kinase inhibitor treatment for 1 week decreased the hyperoxia induced increase of Rho-kinase activity on postnatal d 21. Rho-kinase inhibitor treatment decreased RVH and pulmonary arterial remodeling in hyperoxia-exposed rats. However, the differences did not reach statistical significance. The discrepancy between ours and Enomoto et al studies may be due to different duration of hyperoxia exposure and age of study.
An increasing number of studies implicate oxidant stress as an important initiating factor in pulmonary hypertension27. Oxidant stress and the consequent upregulation of G protein-coupled receptor ligands that are critical to the pathogenesis of pulmonary hypertension are known to activate RhoA28. Recent studies have implicated the small GTPase, RhoA and its effector protein Rho-kinase, as a key pathway regulating pulmonary vascular tone29. We speculate that these reactive oxygen species-induced mediators lead to activation of the RhoA/ROCK pathway in our rat model of pulmonary hypertension.
In summary, we found that exposing neonatal rats to 3-week hyperoxia caused pulmonary hypertension, as shown by increased RVH and arterial MWT. Pulmonary hypertension was associated with increased Rho-kinase activity in the third postnatal week, and Rho-kinase inhibitor treatment for 1 week tended to attenuate RVH. These results demonstrate that Rho-kinase plays an important role in the pathogenesis of hyperoxia-induced pulmonary hypertension and that Rho-kinase might be a potential therapeutic target in attenuating hyperoxia induced structural changes in pulmonary hypertension.
Author contribution
Hsiu-chu CHOU performed histological experiments, analyzed the data, and was responsible for image analysis; Liang-ti HUANG performed animal experiments; Tsu-fu YEH performed part of the research and analyzed the data; and Chung-ming CHEN performed research, coordinated experiments, analyzed the data, wrote the paper, and drafted the manuscript.
Abbreviations
ERM, ezrin, radixin, and moesin; MWT, medial wall thickness; p-MYPT1, phospho-myosin phosphatase target subunit 1; RVH, right ventricular hypertrophy.
References
Ratner V, Slinko S, Utkina-Sosunova I, Starkov A, Polin RA, Ten VS . Hypoxic stress exacerbates hyperoxia-induced lung injury in a neonatal mouse model of bronchopulmonary dysplasia. Neonatology 2009; 95: 299–305.
Jiang JS, Lang YD, Chou HC, Shih CM, Wu MY, Chen CM, et al. Activation of the renin-angiotensin system in hyperoxia-induced lung fibrosis in neonatal rats. Neonatology 2012; 101: 47–54.
Han RN, Buch S, Tseu I, Young J, Christie NA, Frndova H, et al. Changes in structure, mechanics, and insulin like growth factor-related gene expression in the lungs of newborn rats exposed to air or 60% oxygen. Pediatr Res 1996; 39: 921–9.
Vadivel A, Aschner JL, Rey-Parra GJ, Magarik J, Zeng H, Summar M, et al. L-citrulline attenuates arrested alveolar growth and pulmonary hypertension in oxygen-induced lung injury in newborn rats. Pediatr Res 2010; 68: 519–25.
Belik J, Jankov RP, Pan J, Tanswell AK . Chronic O2 exposure enhances vascular and airway smooth muscle contraction in the newborn but not adult rat. J Appl Physiol 2003; 94: 2303–12.
Ladha F, Bonnet S, Eaton F, Hashimoto K, Korbutt G, Thébaud B, et al. Sildenafil improves alveolar growth and pulmonary hypertension in hyperoxia-induced lung injury. Am J Respir Crit Care Med 2005; 172: 750–6.
de Visser YP, Walther FJ, Laghmani el H, Boersma H, van der Laarse A, Wagenaar GT . Sildenafil attenuates pulmonary inflammation and fibrin deposition, mortality and right ventricular hypertrophy in neonatal hyperoxic lung injury. Respir Res 2009; 10: 30.
Amano M, Fukata Y, Kaibuchi K . Regulation and function of Rho associated kinase. Exp Cell Res 2000; 261: 44–51.
Budzyn K, Marley P, Sobey C . Targeting Rho and Rho-kinase in the treatment of cardiovascular disease. Trends Pharmacol Sci 2006; 27: 97–104.
Rikitake Y, Liao JK . ROCKs as therapeutic targets in cardiovascular diseases. Expert Rev Cardiovasc Ther 2005; 3: 441–51.
Jiang B, Tawara S, Abe K, Takaki A, Fukumoto Y, Shimokawa H . Acute vasodilator effect of fasudil, a Rho-kinase inhibitor, in monocrotaline-induced pulmonary hypertension in rats. J Cardiovasc Pharmacol 2007; 49: 85–9.
Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, et al. Rho-kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res 2007; 100: 923–9.
Homma N, Nagaoka T, Karoor V, Imamura M, Tarasevicience-Stewart L, Walker LA, et al. Involvement of RhoA/Rho kinase signaling in protection against monocrotaline-induced pulmonary hypertension in pneumonectomized rats by dehydroepiandrosterone. Am J Physiol Lung Cell Mol Physiol 2008; 295: L71–8.
Xing X, Gan Y, Wu S, Chen P, Zhou R, Xiang X . Rho-kinase as a potential therapeutic target for the treatment of pulmonary hypertension. Drug News Perspect 2006; 19: 517–22.
Ziino AJ, Ivanovska J, Belcastro R, Kantores C, Xu EZ, Lau M, et al. Effects of rho-kinase inhibition on pulmonary hypertension, lung growth, and structure in neonatal rats chronically exposed to hypoxia. Pediatr Res 2010; 67: 177–82.
Xu EZ, Kantores C, Ivanovska J, Engelberts D, Kavanagh BP, McNamara PJ, et al. Rescue treatment with a Rho-kinase inhibitor normalizes right ventricular function and reverses remodeling in juvenile rats with chronic pulmonary hypertension. Am J Physiol Heart Circ Physiol 2010; 299: H1854–64.
Zhong YS, Yu CH, Ying HZ, Wang ZY, Cai HF . Prophylactic effects of Orthosiphon stamineus Benth. extracts on experimental induction of calcium oxalate nephrolithiasis in rats. J Ethnopharmacol 2012; 144: 761–7.
Agard C, Rolli-Derkinderen M, Dumas-de-La-Roque E, Rio M, Sagan C, Savineau JP, et al. Protective role of the antidiabetic drug metformin against chronic experimental pulmonary hypertension. Br J Pharmacol 2009; 158: 1285–94.
Masood A, Yi M, Lau M, Belcastro R, Shek S, Pan J, et al. Therapeutic effects of hypercapnia on chronic lung injury and vascular remodeling in neonatal rats. Am J Physiol 2009; 297: L920–30.
Li XQ, Wang HM, Yang CG, Zhang XH, Han DD, Wang HL . Fluoxetine inhibited extracellular matrix of pulmonary artery and inflammation of lungs in monocrotaline-treated rats. Acta Pharmacol Sin 2011; 32: 217–22.
Burri PH . The postnatal growth of the rat lung. III. Morphology. Anat Rec 1974; 180: 77–98.
Pfitzer G . Regulation of myosin light chain phosphorylation in smooth muscle. J Appl Physiol 2001; 91: 497–503.
Raftopoulou M, Hall A . Cell migration: Rho GTPases lead the way. Dev Biol 2004; 265: 23–32.
Takahara A, Sugiyama A, Satoh Y, Yoneyama M, Hashimoto K . Cardiovascular effects of Y-27632, a selective Rho-associated kinase inhibitor, assessed in the halothane-anesthetized canine model. Eur J Pharmacol 2003; 460: 51–7.
Nagaoka T, Morio Y, Casanova N, Bauer N, Gebb S, McMurtry I, et al. Rho/Rho kinase signaling mediates increased basal pulmonary vascular tone in chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol 2004; 287: L665–72.
Enomoto M, Gosal K, Cubells E, Escobar J, Vento M, Jankov RP, et al. Sex-dependent changes in the pulmonary vasoconstriction potential of newborn rats following short-term oxygen exposure. Pediatr Res 2012; 72: 468–78.
Wedgwood S, Black SM . Role of reactive oxygen species in vascular remodeling associated with pulmonary hypertension. Antioxid Redox Signal 2002; 5: 759–69.
Jin L, Ying Z, Webb RC . Activation of Rho/Rho kinase signaling pathway by reactive oxygen species in rat aorta. Am J Physiol Heart Circ Physiol 2004; 287: H1495–500.
Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, et al. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J 1996; 15: 2208–16.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chou, Hc., Huang, Lt., Yeh, Tf. et al. Rho-kinase inhibitor Y-27632 attenuates pulmonary hypertension in hyperoxia-exposed newborn rats. Acta Pharmacol Sin 34, 1310–1316 (2013). https://doi.org/10.1038/aps.2013.93
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2013.93
Keywords
This article is cited by
-
Progress of Polysaccharide-Contained Polyurethanes for Biomedical Applications
Tissue Engineering and Regenerative Medicine (2022)