Abstract
Aim:
The hypoxic condition within large or infiltrative hypovascular tumors produces intracellular acidification, which could activate many signaling pathways and augment cancer cell growth and invasion. Carbonic anhydrase-IX (CA-IX) is an enzyme lowering pH. This study is to examine whether hypoxia induces CA-IX in hepatocellular carcinoma (HCC) cells, and to evaluate its clinical implication in HCC patients.
Methods:
Human HCC cell lines (Huh-7 and HepG2 cells) were used, and cell growth was assessed using MTS assay. CA-IX expression and apoptotic/kinase signaling were evaluated using immunoblotting. The cells were transfected with CA-IX-specific siRNA, or treated with its inhibitor 4-(2-aminoethyl) benzenesulfonamide (CAI#1), and/or the hexokinase II inhibitor, 3-bromopyruvate (3-BP). A clinic pathological analysis of 69 patients who underwent an HCC resection was performed using a tissue array.
Results:
Incubation of HCC cells under hypoxia (1% O2, 5% CO2, 94% N2) for 36 h significantly increased CA-IX expression level. CAI#1 (400 μmol/L) or CA-IX siRNA (100 μmol/L) did not influence HCC cell growth and induce apoptosis. However, CAI#1 or CA-IX siRNA at these concentrations enhanced the apoptosis induced by 3-BP (100 μmol/L). This enhancement was attributed to increased ER stress and JNK activation, as compared with 3-BP alone. Furthermore, a clinic pathological analysis of 69 HCC patients revealed that tumor CA-IX intensity was inversely related to E-cadherin intensity.
Conclusion:
Inhibition of hypoxia-induced CA-IX enhances hexokinase II inhibitor-induced HCC apoptosis. Furthermore, CA-IX expression profiles may have prognostic implications in HCC patients. Thus, the inhibition of CA-IX, in combination with a hexokinase II inhibitor, may be therapeutically useful in patients with HCCs that are aggressively growing in a hypoxic environment.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) is one of the most aggressive malignancies originating from the diseased liver1, 2, 3. HCCs are characteristically hypervascular; therefore, transarterial chemoembolization (TACE) is considered one of the favorable treatment options for unresectable HCCs. However, cells that survive in HCC nodules despite TACE treatment, which confers a robust hypoxic insult, sometimes grow more rapidly than those in neighboring nodules4. Moreover, HCCs occasionally exhibit an infiltrating rather than mass-forming growth pattern5, 6, and these advanced infiltrative HCCs seldom show hypervascularity, grow more rapidly, and have a poorer prognosis than mass-forming hypervascular tumors. Therefore, hypoxia seems to promote signals that allow HCC cells to survive and proliferate in a hypoxic environment7.
In the hypoxic state, a glycolytic system replaces oxidative phosphorylation as a salvage pathway for generating adenosine triphosphate7. Many glycolytic enzymes and glucose transporters are induced by hypoxia inducible factor-1 (HIF-1) through hypoxia response elements in their promoters8. HIF-1 also induces the two lactate dehydrogenase isoforms, LDH-5 and LDH-A, and pyruvate dehydrogenase kinase 1. These lactate dehydrogenase isoforms convert pyruvate to lactate, and pyruvate dehydrogenase kinase 1 prevents pyruvate from entering the tricarboxylic acid cycle, which enables the switch to a glycolytic metabolism8. Furthermore, hexokinase (HK) is the first enzyme in this pathway and is essential for maintaining the high glycolytic phenotype9. We previously demonstrated that hypoxia stimulates HCC cell growth by inducing HK II expression7, and in vivo HK II inhibition reveals an anti-tumor effect through the induction of apoptosis10. Glycolysis produces excessive amounts of lactate and carbon dioxide (CO2) as by-products. However, these waste products are not efficiently removed, due to the high tumor interstitial pressure and defective vasculature11, 12, and the resulting acidic milieu causes transient intracellular acidification, which is incompatible with cell growth and survival. Thus, tumor cells that are either located within the hypovascular tumor or reside in the center of large tumors are exposed to a hypoxia-induced acidic microenvironment; however, cancer cells adapt to this acidic setting and continue to grow.
Carbonic anhydrase-IX (CA-IX) is a transmembrane protein with a catalytic site in the extracellular space, and it is involved in lowering pH by expediting the pericellular metabolism of CO2 in a collaboration with bicarbonate transporters13. In response to hypoxia, HIF-1 directly activates CA9 gene transcription and up-regulates CA-IX protein expression8. Although CA-IX is expressed in few normal tissues, it is expressed in many cancers, and its overexpression was reported to be related to poor prognosis14, 15. Based on this knowledge, we postulated that CA-IX is one of the possible mechanisms by which HCC adapts to the acidic tumor milieu. Therefore, in this study, we aimed to examine whether CA-IX is induced by hypoxia in HCC cells, and we evaluated its clinical implications in HCC patients.
Materials and methods
Cell culture
Huh-7 and HepG2 cells, which were derived from a well-differentiated HCC, were used in this study. Cells were grown in DMEM supplemented with 10% fetal bovine serum, streptomycin (100 mg/L), and penicillin (100 U/mL). Cell proliferation assays were performed using 3% fetal bovine serum, and the other experiments were performed using cells that were serum-starved overnight to avoid serum inducing signals. Depending on the specific experiment, cells were incubated either under standard culture conditions (20% O2 and 5% CO2 at 37 °C) or under hypoxic conditions (1% O2, 5% CO2, and 94% N2 at 37 °C).
Chemicals and reagents
3-Bromopyruvate (3-BP) and carbonic anhydrase inhibitor (CAI) #1 sulfonamide [4-(2-aminoethyl)-benzenesulfonamide] were obtained from Sigma-Aldrich, Inc (St Louis, MO, USA). SP600125 (a c-Jun NH2-terminal kinase (JNK) inhibitor) was obtained from Biomol Research Laboratories (Plymouth Meeting, PA, USA).
Cell proliferation
The CellTiter 96 Aqueous One Solution cell proliferation assay (Promega, Madison, WI, USA) was used to measure cell proliferation. In this assay, dehydrogenase enzymes convert the colorimetric MTS reagent [3,4-(5-dimethylthiazole-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium salt] into soluble formazan in only the metabolically active and proliferating cells. After each treatment, 20 μL of dye solution was added to each well of a 96-well plate, which was then incubated for 2 h. Afterward, the 490 nm absorbance was measured with an ELISA plate reader (Molecular Devices, Sunnyvale, CA, USA).
Quantitation of apoptosis
The levels of apoptosis were evaluated using the nuclear binding dye 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) to measure apoptotic cells by fluorescence microscopy (Zeiss, Germany). The cells were treated with DAPI for 30 min and evaluated by fluorescence microscopy. Apoptotic cells were defined as those containing nuclear fragmentation and condensed chromatin. The percentage of apoptotic cells was calculated as the ratio of apoptotic cells to total counted cells ×100. For each treatment, a minimum of 400 cells was counted.
Immunoblot assay
Cells were lysed for 20 min on ice in lysis buffer (50 mmol/L Tris-HCl, pH 7.4, 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L Na3VO4, 1 mmol/L NaF, and 1 μg/mL each of aprotinin, leupeptin, and pepstatin), and they were centrifuged at 14 000×g for 10 min at 4 °C. Samples were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes, blotted with appropriate primary antibodies, and incubated with peroxidase-conjugated secondary antibodies (Biosource International, Camarillo, CA, USA). Bound antibodies were visualized using a chemiluminescent substrate (ECL; Amersham, Arlington Heights, IL, USA) and exposed to Kodak X-OMAT film. The primary antibodies used included mouse anti-CA-IX and mouse anti-phospho-eukaryotic initiation factor 2α (eIF2α), which were obtained from Santa Cruz Biotechnology, Inc (Santa Cruz, CA, USA), and rabbit anti-caspase 7, rabbit anti-caspase 8, rabbit anti-caspase 9, mouse anti-phospho-JNK, rabbit anti-phospho-p42/44, and rabbit anti-phospho-Akt, which were obtained from Cell Signaling Technology Inc (Danvers, MA, USA). Rabbit anti-E-cadherin was obtained from BD Transduction Laboratories (San Jose, CA, USA). An image analyzer (LAS-1000; Fuji Photo Film, Tokyo, Japan) was used to detect images, and densitometric analyses were performed using Image Gauge software (Fuji Photo Film). Arbitrary units were calculated by densitometric scanning of the intensity of CA-IX relative to actin intensity, setting the 0 h data point to 1.
Small interfering RNA (siRNA) transfection
CA-IX-specific siRNA was obtained from Dharmacon (Lafayette, CO, USA). Huh-7 and HepG2 cells were transfected with CA-IX-specific siRNA and incubated at concentrations ranging from 0 to 200 μmol/L, in a hypoxic state. Cells were lysed after 24 h, and immunoblot analysis was performed with anti-CA-IX and anti-actin antibodies.
Tissue array and immunohistochemical analyses of surgical specimens
We retrospectively evaluated HCC tissue specimens from 69 HCC patients (male, 89.9%; mean age 52±9 years) who had undergone TACE followed by surgical resection at Seoul National University Hospital, Seoul, Korea, between June 1994 and December 1998. The study protocol was approved by the Institutional Review Board of Seoul National University Hospital.
Tumor specimens from each patient were processed into 10% neutral formalin fixed, paraffin-embedded blocks. Tumor staging was evaluated in accordance with the American Joint Committee on Cancer (AJCC) staging system, 6th edition16. All tumors were histologically diagnosed, graded according to Edmondson's scale, and grouped as either low grade (I or II) or high grade (III or IV). Tumors were also stratified by multiplicity and the presence or absence of microscopic vascular invasion. For immunohistochemical staining, all specimens were evaluated using a tissue-array method. Core tissue biopsies (2 mm in diameter) were taken from individual paraffin embedded tissues (donor blocks) and arranged in a new recipient paraffin block (tissue array block) using a trephine apparatus (Superbiochips Laboratories, Seoul, Korea). Immunohistochemical staining was performed with E-cadherin (BD Transduction Laboratories) and CA-IX (Santa Cruz Biotechnology Inc) antibodies, using a streptavidin peroxidase-based procedure after microwave antigen retrieval. The immunoreactive intensity for each case was scored as none, weak, moderate, or strong. The extent of immunoreactivity was scored as less than one-third or more than one-third for E-cadherin, and it was scored as less than 10%, 10% to 50%, or more than 50% for CA-IX. Histological examinations were performed by an experienced pathologist (Ja-june JANG) who was unaware of any clinical information.
Statistical analysis
All cell-based experimental data were acquired from at least three independent experiments, with a minimum of three separate isolations, and were expressed as the mean±standard deviation (SD). Statistical evaluations of numeric variables in each group were conducted using the Mann-Whitney U test. The clinicopathological data were analyzed using the chi-square test. All statistical analyses were performed using SPSS version 17.0 (SPSS, Inc, Chicago, IL, USA). Statistical significance was defined as a P value less than 0.05.
Results
Hypoxia induced CA-IX expression in HCC cells
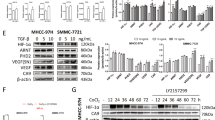
We first investigated whether CA-IX expression is hypoxia-inducible in HCC cells. For this purpose, the two different human HCC cell lines were serum starved and cultured in a hypoxic state. Hypoxia increased CA-IX expression in both Huh-7 and HepG2 cells (Figure 1A). This finding suggests that HCC cells do express CA-IX and that its expression is hypoxia-inducible in different cell lines.
Hypoxia-induced CA-IX expression and the effect of its inhibition in human HCC cell lines. (A) Hypoxia increased CA-IX expression in HCC cells. Huh-7 and HepG2 cells were cultured under hypoxic conditions for the indicated times. Equivalent amounts of proteins were immunoblotted using anti-CA-IX and anti-actin antibodies. Densitometric analyses were performed, and data are expressed as the mean±SD of relative intensity ratios of CA-IX to actin. bP<0.05 vs 24 h. (B) CA-IX expression was efficiently reduced by CA-IX-specific siRNA in a dose-dependent manner. Huh-7 and HepG2 cells were transfected with CA-IX siRNA for 24 h and incubated at concentrations ranging from 0 to 200 μmol/L in a hypoxic state. Cells were lysed after 24 h, and immunoblot analysis was performed using anti-CA-IX and anti-actin antibodies. Densitometric analyses were performed and data were expressed as the mean±SD of relative intensity ratios of CA-IX to actin. bP<0.05 vs 0 μmol/L. (C) Inhibition of CA-IX expression failed to influence the HCC cell growth. Huh-7 and HepG2 cells were treated with either CA-IX siRNA (0 and 100 μmol/L) or CAI#1 (0 and 1 mmol/L) for 24 h with 3% fetal bovine serum under normoxic or hypoxic culture conditions. Cell growth was determined using the MTS assay. (D) Inhibition of CA-IX expression failed to modulate HCC cell apoptosis. Huh-7 and HepG2 cells were serum starved for 15 h and then treated with CA-IX siRNA (0 and 100 μmol/L) or CAI#1 (0 and 400 μmol/L). Apoptotic cells were DAPI stained, visualized by fluorescent microscopy, and counted after 4 and 6 h of hypoxia, respectively. Data were expressed as the mean±SD. siRNA, CA-IX siRNA; N, normoxia; H, hypoxia.
The effect of CA-IX inhibition on HCC cellular growth or apoptotic cell death
To inhibit CA-IX, either its expression was suppressed by siRNA transfection (Figure 1B) or its activity was inhibited by sulfonamide, a carbonic anhydrase inhibitor #1 (CAI#1). Both CA-IX siRNA and CAI#1 treatment failed to suppress HCC cell growth under normoxic or hypoxic culture conditions (P>0.05) (Figure 1C). In addition, neither CA-IX siRNA nor CAI#1 treatment significantly induced HCC cell apoptosis under hypoxic culture conditions (P>0.05) (Figure 1D). These observations indicate that changes in CA-IX expression or activity do not affect HCC cell growth or survival.
Enhanced HK II inhibitor-induced apoptosis by CA-IX inhibition in HCC cells
Because hypoxia induces both HK II7 and CA-IX13 expression through HIF-1, we hypothesized that simultaneously blocking these two enzymes would enhance 3-BP-induced apoptosis in HCC cells under hypoxic conditions. When HCC cells that were CA-IX-inhibited with either siRNA or CAI#1 were treated with 3-BP under hypoxic conditions, apoptosis was significantly enhanced compared to cells treated with 3-BP alone (Figure 2A). However, this enhancement was not observed in cells similarly treated under normoxic culture conditions (Figure 2B). Thus, these findings demonstrate that the enhancement of 3-BP-induced HCC cell apoptosis by CA-IX inhibition is hypoxia specific.
Effect of CA-IX inhibition on 3-BP-induced apoptosis in hypoxic HCC cells. (A) CA-IX inhibition enhances 3-BP-induced apoptosis in hypoxic HCC cells. Huh-7 and HepG2 cells were serum starved for 15 h and then treated with 3-BP (100 μmol/L) in the presence or absence of either CA-IX siRNA (0 and 100 μmol/L) or CAI#1 (0 and 400 μmol/L) for 24 h. Apoptotic cells were counted by DAPI staining and fluorescent microscopy after 4 and 6 h of hypoxia, respectively. (B) Apoptosis enhancement by CA-IX inhibition is a hypoxia-specific phenomenon. Huh-7 and HepG2 cells were serum starved for 24 h either under normoxic or hypoxic conditions and then treated with 3-BP (100 μmol/L) in the presence or absence of CAI#1 (0 and 1 mmol/L) for 5 h. Apoptotic cells were then counted by DAPI staining and fluorescent microscopy. (C) The activation of caspases 7 and 9 was more prominent in 3-BP/CAI#1-treated cells than in cells treated with 3-BP alone, and (D) CA-IX inhibition induces ER stress-dependent JNK activation. Huh-7 cells were serum starved, cultured under hypoxic conditions, and then treated with 3-BP (100 μmol/L) in the presence or absence of CAI#1 (0 and 1 mmol/L) for the indicated times. Cells were lysed and immunoblot analysis was performed using anti-caspase 7, anti-caspase 8, anti-caspase 9, anti-phospho-JNK, anti-phospho-p42/44, anti-phospho-Akt, anti-phospho-eIF2α, and anti-actin antibodies. (E) JNK activation participates in 3-BP-induced apoptosis enhancement by CA-IX inhibition. Huh-7 cells were serum starved for 24 h under hypoxic culture conditions and then treated with 3-BP (0 and 100 μmol/L) in the presence or absence of CAI#1 (0 and 1 mmol/L) and/or JNK inhibitor (0 and 10 μmol/L) for 6 h. Apoptotic cells were counted by DAPI staining and fluorescent microscopy. Data were expressed as the mean±SD. bP<0.05. siRNA, CA-IX siRNA; J–I, JNK inhibitor.
Mechanism of apoptosis enhancement by CA-IX inhibition
To explore possible mechanisms of enhanced 3-BP-induced apoptosis through CA-IX inhibition, we next explored which apoptotic signaling pathway was more activated in 3-BP/CAI#1-treated cells compared to cells treated with 3-BP alone. When cells were treated with both 3-BP and CAI#1, the activation of caspases 9 and 7 was more prominent than in cells treated with 3-BP alone (Figure 2C), indicating that the activation of the mitochondrial apoptotic signaling pathway is enhanced by CA-IX inhibition. We then explored kinase signals that are known to regulate apoptosis and found that pro-apoptotic JNK was more promptly and potently activated in cells treated with 3-BP and CAI#1 than in cells treated with 3-BP alone, whereas prosurvival signals such as p42/44 and Akt were unaffected (Figure 2D). Because JNK activation might depend on endoplasmic reticulum (ER) stress, we evaluated whether ER stress is activated in 3-BP and CAI#1-treated cells. Indeed, eIF2α phosphorylation, which indicates ER stress activation, was prominent in these cells (Figure 2D). Finally, we examined whether JNK activation participated in 3-BP-induced apoptosis enhancement by CA-IX inhibition and found that JNK inhibition did attenuate this enhancement (Figure 2E). Therefore, these findings collectively suggest that CA-IX inhibition may induce ER stress-dependent JNK activation in 3-BP-treated HCC cells and thus enhance apoptotic cell death in these cells.
Prognostic implications of CA-IX expression in HCC patients
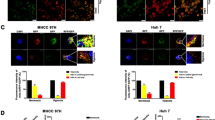
Because we found that CA-IX modulation may be therapeutically important in HCC cells, particularly under hypoxic conditions, and could also confer a potent survival signal to cells, we next evaluated whether CA-IX is expressed in human HCC tissues and, if so, whether its expression is linked to patient prognosis. For this purpose, we employed a tissue array and performed clinicopathological analysis using HCC tissues from 69 patients who had undergone TACE prior to surgical resection because TACE may powerfully activate hypoxia-induced signals in HCC cells. Of the 69 specimens, 48, 17, and 4 cases showed none, weak, or moderate CA-IX cell membrane immunoreactivity, respectively. Representative cases are shown in Figure 3A. The clinicopathological findings are summarized in Table 1. Following clinicopathological analysis, there was no correlation between the extent of CA-IX immunoreactivity and clinicopathological variables (Table 2). However, we found that tumoral CA-IX intensity was inversely related to E-cadherin intensity (Figure 3B, Table 3). E-cadherin is representative of classic cadherins, and its down-regulation has been frequently associated with invasiveness, metastasis, and a poor prognosis in a variety of human cancers, including HCC17, 18. This finding suggests that CA-IX expression in HCC tissues may have prognostic implications in HCC patients.
CA-IX expression in HCC tumor tissues and its prognostic implications in HCC patients. (A) Representative immunohistochemical staining showed (a) no, (b) weak, and (c) moderate CA-IX immunoreactivity in human HCC tumor tissues. Original magnification ×400. (B) HCC tumoral CA-IX intensity is inversely related to E-cadherin intensity. Statistical significance was analyzed using the chi-square test. P=0.024. (C) E-cadherin expression increases when CA-IX is inhibited. Huh-7 cells were transfected with CA-IX siRNA for 24 h and incubated at concentrations ranging from 0 to 200 μmol/L in a hypoxic state for 24 h. Cells were then lysed, and immunoblot analysis was performed using anti-E-cadherin and anti-actin antibodies. E-cad, E-cadherin.
Decreased E-cadherin expression by CA-IX in HCC cells
We next evaluated whether E-cadherin expression levels are affected by modulation of CA-IX expression in vitro. When CA-IX expression was suppressed by siRNA in HCC cells, E-cadherin expression increased (Figure 3C). This finding is compatible with that of a previous study, in which CA-IX was found to decrease E-cadherin levels in the Madin-Darby Canine kidney cell line19, and our results show that tumoral CA-IX intensity is inversely related to E-cadherin intensity. Altogether, these findings suggest that the induction of CA-IX expression may attenuate E-cadherin expression, thus leading to epithelial-mesenchymal transition potentiating invasiveness and metastasis.
Discussion
The principal findings of this study relate to hypoxia-induced activation of an intracellular pH maintenance mechanism in human HCC cells. Specifically, this study demonstrates that hypoxia stimulates the expression of CA-IX in HCC cells. Furthermore, although CA-IX inhibition alone failed to induce significant HCC cell growth suppression or apoptosis, CA-IX inhibition enhanced 3-BP-induced apoptotic cell death mediated by ER stress-dependent JNK activation. In addition, HCC tissues express CA-IX, and tumoral CA-IX intensity is inversely related to E-cadherin intensity, thus potentiating invasiveness and metastasis. Each of these findings is discussed in further detail below.
Among CA isoenzymes, it has become clear that CA-IX is the most important enzyme for tumorigenesis and prognostication in various tumors including pancreatic20 and rectal15 cancers. In the liver, it has been reported that 78% of cholangiocarcinomas show a positive reaction for CA-IX, whereas HCCs show a weak immunoreactivity in only 33% of cases21. Furthermore, in contrast to other malignancies, the prognostic role of CA-IX in HCC has not been fully elucidated. In the present study, we observed that CA-IX is expressed in different HCC cell lines under hypoxic culture conditions. This finding suggests that CA-IX may participate in intracellular pH maintenance under innate intratumoral hypoxic conditions or during hypoxic insult such as that induced by TACE. However, HCC cell death or growth was not significantly affected by CA-IX inhibition alone. This can be explained by the fact that tumor cells can regulate their cytoplasmic pH via the Na+/H+ exchanger, the H+/monocarboxylate transporter, and the vacuolar H+/ATP pump in addition to CA-IX8.
Because hypoxia induces both HK II7 and CA-IX13 expression through HIF-1, we postulated that simultaneously blocking these two enzymes could enhance 3-BP-induced apoptosis in HCC cells. Indeed, we confirmed that 3-BP-induced HCC cell apoptosis was significantly enhanced by CA-IX inhibition and that this enhancement was hypoxia specific. This finding indicates that a combinatorial strategy is more effective for HCCs under hypoxic conditions, such as in advanced infiltrative HCCs, which seldom show hypervascularity, grow more rapidly, and have a poorer prognosis than mass-forming hypervascular subtypes.
We explored the possible mechanism of apoptosis enhancement by CA-IX inhibition and found that the activation of caspases 9 and 7 by 3-BP/CAI#1 was more prominent than the activation induced by 3-BP alone, which suggests that activation of mitochondrial apoptotic signaling is augmented by CA-IX inhibition. In addition, we found that the proapoptotic JNK was more promptly and potently activated in cells treated with both 3-BP and a CA-IX inhibitor compared to cells treated with 3-BP alone. JNK performs a crucial role in modulating the function of proapoptotic proteins placed in the mitochondria22. JNK has been shown to be required for the release of cytochrome c from the inner membrane space of mitochondria. Released cytochrome c, in combination with apoptotic peptidase activating factor 1 and caspase 9, forms apoptosomes that trigger the caspase 9 cascade23. It has been previously reported that phosphorylated CA-IX could cooperate with the regulatory subunit of phosphatidylinositol 3 kinase and induce Akt activation, which could participate in cancer progression24. However, in contrast to this previous study, phosphorylation of both the prosurvival Akt and p42/44 MAPK were not affected by 3-BP or 3-BP+CA-IX inhibitor treatment in the present study.
In a recent study, it was reported that 3-BP may induce ER stress and thereby cause apoptosis in human HCC cell lines25. Because ER stress induction may activate JNK8, 26, we next evaluated whether ER stress is more activated in cells treated with 3-BP and a CA-IX inhibitor than in cells treated with 3-BP alone, and we found that eIF2α phosphorylation was more prominent in cells treated with 3-BP and a CA-IX inhibitor. ER stress sensors initiate an unfolded protein response (UPR) that results in the phosphorylation of its main substrate, eIF2α, and direct binding of the eIF2α-dependent translationally regulated gene activating transcription factor 4 to the CA9 promoter27. These data suggest that 3-BP-induced ER stress can trigger CA-IX expression as one of the UPRs that reduces ER stress. Thus, the simultaneous inhibition of CA-IX and HK II may disturb ER stress alleviation, thereby leading to ER stress escalation, subsequent JNK activation, and apoptosis enhancement through the mitochondrial activation of caspases 9 and 7. Indeed, in the present study, we observed that JNK inhibition attenuated apoptosis enhancement.
Acidic extracellular pH inhibits the binding of cells to CA-IX, suggesting that the CA-IX adhesion ability can be modulated by the tumor milieu28. CA-IX has also been implicated in intercellular adhesion19. In this previous study, it was found that CA-IX is localized with the key adhesion molecule E-cadherin, internalizes in response to the same external stimuli as E-cadherin, and reduces cell-to-cell adhesion by destabilizing the association between E-cadherin and the cytoskeleton through a method that involves direct binding of CA-IX to β-catenin19. In the present study, tumoral CA-IX intensity was found to be inversely related to E-cadherin intensity, increases of E-cadherin intensity may predict a favorable prognosis29. Furthermore, when CA-IX expression was suppressed by siRNA, E-cadherin expression was increased. The ability of CA-IX to adhere to β-catenin19 is consistent with the suggestion that hypoxia can promote tumor invasion by decreasing E-cadherin-mediated intercellular adhesion, offering the possibility that CA-IX participates in epithelial-mesenchymal transition30, 31. Moreover, an alkaline intracellular pH and an acidic extracellular pH have been hypothesized to increase tumor growth32. Thus, in CA-IX-expressing HCCs, CA-IX inhibition could be therapeutically useful for reducing tumor survival or invasiveness and metastasis.
This study demonstrates that the inhibition of hypoxia-inducible CA-IX enhances 3-BP-induced HCC cell apoptosis and that CA-IX expression profiles may have prognostic implications in HCC patients. Thus, the blockage of CA-IX in combination with hexokinase II inhibitor treatment may be therapeutically useful in patients with large or infiltrative hypovascular HCCs that are aggressively growing in a hypoxic environment.
Author contribution
Su-jong YU and Jung-hwan YOON designed the research; Su-jong YU, Eun-sun JANG, Min-sun KWAK, and Eun-ju CHO performed the research; Su-jong YU, Sun-jung MYUNG, and Jung-hwan YOON analyzed the data; Ja-june JANG performed histological examinations; Jung-hwan YOON, Jeong-hoon LEE, Yoon-jun KIM, and Hyo-suk LEE critically revised the manuscript for important intellectual content; Su-jong YU wrote the paper.
References
Liang TJ, Jeffers LJ, Reddy KR, de Medina M, Parker IT, Cheinquer H, et al. Viral pathogenesis of hepatocellular carcinoma in the United States. Hepatology 1993; 18: 1326–33.
Colombo M, de Franchis R, Del Ninno E, Sangiovanni A, de Fazio C, Tommasini M, et al. Hepatocellular carcinoma in Italian patients with cirrhosis. N Engl J Med 1991; 325: 675–80.
Koike K . Role of hepatitis viruses in multistep hepatocarcinogenesis. Dig Liver Dis 2001; 33: 2–6.
Tezuka M, Hayashi K, Kubota K, Sekine S, Okada Y, Ina H, et al. Growth rate of locally recurrent hepatocellular carcinoma after transcatheter arterial chemoembolization: comparing the growth rate of locally recurrent tumor with that of primary hepatocellular carcinoma. Dig Dis Sci 2007; 52: 783–8.
Trevisani F, Caraceni P, Bernardi M, D'Intino PE, Arienti V, Amorati P, et al. Gross pathologic types of hepatocellular carcinoma in Italian patients. Relationship with demographic, environmental, and clinical factors. Cancer 1993; 72: 1557–63.
Weinstein-Oppenheimer CR, Henriquez-Roldan CF, Davis JM, Navolanic PM, Saleh OA, Steelman LS, et al. Role of the Raf signal transduction cascade in the in vitro resistance to the anticancer drug doxorubicin. Clin Cancer Res 2001; 7: 2898–907.
Gwak GY, Yoon JH, Kim KM, Lee HS, Chung JW, Gores GJ . Hypoxia stimulates proliferation of human hepatoma cells through the induction of hexokinase II expression. J Hepatol 2005; 42: 358–64.
Pastorekova S, Zatovicova M, Pastorek J . Cancer-associated carbonic anhydrases and their inhibition. Curr Pharm Des 2008; 14: 685–98.
Bustamante E, Morris HP, Pedersen PL . Energy metabolism of tumor cells. Requirement for a form of hexokinase with a propensity for mitochondrial binding. J Biol Chem 1981; 256: 8699–704.
Kim W, Yoon JH, Jeong JM, Cheon GJ, Lee TS, Yang JI, et al. Apoptosis-inducing antitumor efficacy of hexokinase II inhibitor in hepatocellular carcinoma. Mol Cancer Ther 2007; 6: 2554–62.
Helmlinger G, Sckell A, Dellian M, Forbes NS, Jain RK . Acid production in glycolysis-impaired tumors provides new insights into tumor metabolism. Clin Cancer Res 2002; 8: 1284–91.
Fukumura D, Jain RK . Tumor microenvironment abnormalities: causes, consequences, and strategies to normalize. J Cell Biochem 2007; 101: 937–49.
Pastorekova S, Ratcliffe PJ, Pastorek J . Molecular mechanisms of carbonic anhydrase IX-mediated pH regulation under hypoxia. BJU Int 2008; 101: 8–15.
Swinson DE, Jones JL, Richardson D, Wykoff C, Turley H, Pastorek J, et al. Carbonic anhydrase IX expression, a novel surrogate marker of tumor hypoxia, is associated with a poor prognosis in non-small-cell lung cancer. J Clin Oncol 2003; 21: 473–82.
Korkeila E, Talvinen K, Jaakkola PM, Minn H, Syrjanen K, Sundstrom J, et al. Expression of carbonic anhydrase IX suggests poor outcome in rectal cancer. Br J Cancer 2009; 100: 874–80.
Greene FL, American Joint Committee on Cancer, American Cancer Society. AJCC cancer staging handbook : from the AJCC cancer staging manual. 6th ed. New York Springer; 2002.
Endo K, Ueda T, Ueyama J, Ohta T, Terada T . Immunoreactive E-cadherin, alpha-catenin, beta-catenin, and gamma-catenin proteins in hepatocellular carcinoma: relationships with tumor grade, clinicopathologic parameters, and patients' survival. Hum Pathol 2000; 31: 558–65.
Berx G, Cleton-Jansen AM, Nollet F, de Leeuw WJ, van de Vijver M, Cornelisse C, et al. E-cadherin is a tumour/invasion suppressor gene mutated in human lobular breast cancers. EMBO J 1995; 14: 6107–15.
Svastova E, Zilka N, Zat'ovicova M, Gibadulinova A, Ciampor F, Pastorek J, et al. Carbonic anhydrase IX reduces E-cadherin-mediated adhesion of MDCK cells via interaction with beta-catenin. Exp Cell Res 2003; 290: 332–45.
Kivela AJ, Parkkila S, Saarnio J, Karttunen TJ, Kivela J, Parkkila AK, et al. Expression of transmembrane carbonic anhydrase isoenzymes IX and XII in normal human pancreas and pancreatic tumours. Histochem Cell Biol 2000; 114: 197–204.
Saarnio J, Parkkila S, Parkkila AK, Pastorekova S, Haukipuro K, Pastorek J, et al. Transmembrane carbonic anhydrase, MN/CA IX, is a potential biomarker for biliary tumours. J Hepatol 2001; 35: 643–9.
Aoki H, Kang PM, Hampe J, Yoshimura K, Noma T, Matsuzaki M, et al. Direct activation of mitochondrial apoptosis machinery by c-Jun N-terminal kinase in adult cardiac myocytes. J Biol Chem 2002; 277: 10244–50.
Dhanasekaran DN, Reddy EP . JNK signaling in apoptosis. Oncogene 2008; 27: 6245–51.
Dorai T, Sawczuk IS, Pastorek J, Wiernik PH, Dutcher JP . The role of carbonic anhydrase IX overexpression in kidney cancer. Eur J Cancer 2005; 41: 2935–47.
Ganapathy-Kanniappan S, Geschwind JF, Kunjithapatham R, Buijs M, Syed LH, Rao PP, et al. 3-Bromopyruvate induces endoplasmic reticulum stress, overcomes autophagy and causes apoptosis in human HCC cell lines. Anticancer Res 2010; 30: 923–35.
Szegezdi E, Logue SE, Gorman AM, Samali A . Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep 2006; 7: 880–5.
van den Beucken T, Magagnin MG, Savelkouls K, Lambin P, Koritzinsky M, Wouters BG . Regulation of Cited2 expression provides a functional link between translational and transcriptional responses during hypoxia. Radiother Oncol 2007; 83: 346–52.
Zavadova Z, Zavada J . Carbonic anhydrase IX (CA IX) mediates tumor cell interactions with microenvironment. Oncol Rep 2005; 13: 977–82.
Lin Q, Li M, Shen ZY, Xiong LW, Pan XF, Gen JF, et al. Prognostic impact of vascular endothelial growth factor-A and E-cadherin expression in completely resected pathologic stage I non-small cell lung cancer. Jpn J Clin Oncol 2010; 40: 670–6.
Beavon IR . Regulation of E-cadherin: does hypoxia initiate the metastatic cascade? Mol Pathol 1999; 52: 179–88.
Sanchez-Tillo E, Lazaro A, Torrent R, Cuatrecasas M, Vaquero EC, Castells A, et al. ZEB1 represses E-cadherin and induces an EMT by recruiting the SWI/SNF chromatin-remodeling protein BRG1. Oncogene 2010; 29: 3490–500.
Martinez-Zaguilan R, Seftor EA, Seftor RE, Chu YW, Gillies RJ, Hendrix MJ . Acidic pH enhances the invasive behavior of human melanoma cells. Clin Exp Metastasis 1996; 14: 176–86.
Acknowledgements
This study was funded, in part, by the Korea Health 21 R&D Project [No 0412-CR01-0704-0001], by a Seoul National University Hospital Research Grant [03-2010-021-0], and by a grant from the Liver Research Foundation of Korea. The authors thank Ms Sung-hee LEE and Ms Soo-mi LEE for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, Sj., Yoon, Jh., Lee, Jh. et al. Inhibition of hypoxia-inducible carbonic anhydrase-IX enhances hexokinase II inhibitor-induced hepatocellular carcinoma cell apoptosis. Acta Pharmacol Sin 32, 912–920 (2011). https://doi.org/10.1038/aps.2011.24
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2011.24
Keywords
This article is cited by
-
Hypoxia-induced reprogramming of glucose-dependent metabolic pathways maintains the stemness of human bone marrow-derived endothelial progenitor cells
Scientific Reports (2023)
-
Carbonic anhydrase-IX inhibition enhances the efficacy of hexokinase II inhibitor for hepatocellular carcinoma in a murine model
Journal of Bioenergetics and Biomembranes (2019)
-
The anticancer agent 3-bromopyruvate: a simple but powerful molecule taken from the lab to the bedside
Journal of Bioenergetics and Biomembranes (2016)
-
Expression of carbonic anhydrase 9 is a novel prognostic marker in resectable hepatocellular carcinoma
Virchows Archiv (2015)
-
Expression of proteins associated with hypoxia and Wnt pathway activation is of prognostic significance in hepatocellular carcinoma
Virchows Archiv (2015)