Abstract
Aim:
To investigate the effect of isochaihulactone (also known as K8), a lignan compound of Bupleurum scorzonerifolium, on H2O2-induced cytotoxicity in neuronally differentiated PC12 cells (nPC12).
Methods:
Viability of neuronal PC12 cells was measured using MTT assay. Protein expression was determined by Western blot. Apoptotic cells was determined using TUNEL assay. D-galactose aging mice were used as a model system to study the anti-oxidant effects of isochaihulactone in vivo.
Results:
Pretreatment with isochaihulactone (5–10 μmol/L) increased cell viability and decreased membrane damage, generation of reactive oxygen species and degradation of poly (ADP-ribose) polymerase in H2O2-treated nPC12 cells and also decreased the expression of cyclooxygenase-2, via downregulation of NF-kappaB, resulting in a decrease in lipid peroxidation. The results suggest that isochaihulactone is a potential antioxidant agent. In a murine aging model, in which chronic systemic exposure to D-galactose (D-gal) causes the acceleration of senescence, administration of isochaihulactone (10 mg·kg-1·d-1, sc) for 7 weeks concomitant with D-gal injection significantly increased superoxide dismutase and glutathione peroxidase activities and decreased the MDA level in plasma. Furthermore, H&E staining to quantify cell death within hippocampus showed that percentage of pyknotic nuclei in the D-gal-treated mice were much higher than in control.
Conclusion:
The results suggest that isochaihulactone exerts potent anti-aging effects against D-gal in mice possibly via antioxidative mechanisms.
Similar content being viewed by others
Introduction
Oxidative stress is believed to be a primary factor in neurodegenerative diseases as well as in the normal process of aging1, 2, 3. Oxygen-derived free radicals exert detrimental effects including peroxidation of membrane lipids, enzyme inactivation, DNA fragmentation and activation of apoptosis4, 5, 6. Superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) act by scavenging the superoxide anion and H2O2 to prevent reactive oxygen species (ROS)-induced damage7. Exogenous H2O2 can increase oxidative stress and apoptotic cell death by causing mitochondrial dysfunction and activation of caspases. Therefore, H2O2 has been used extensively as an inducer of oxidative stress in in vitro models.
Reactive oxygen species themselves can increase and/or induce cellular cyclooxygenase-2 (COX-2) expression8, 9, 10. In addition, apoptotic cell death induced by exposure to cyanide can be inhibited by selective COX-2 inhibition11, 12. Oxidative stress-induced COX-2 expression can be prevented in numerous cell types, including neurons, by free radical scavengers. Thus, oxidant stressors are specific and important inducers of COX-2 gene expression.
The free radical theory of aging was conceived by Harman in 1956. Increasing evidence has convinced many researchers that oxidants play an important role in aging. Chronic administration of a low dose of D-galactose (D-gal) induces changes that resemble natural aging in animals13, 14, 15, 16, 17, 18, 19, 20. D-galactose is a physiological nutrient obtains from lactose in milk. The hydrolysis of lactose in the intestine results monosaccharide glucose and galactose. In animals, galactose is normally metabolized by D-galactokinase and galactose-1-phosphate uridyltransferase but over-supply of D-galactose results its abnormal metabolism (Kaplan and Pesce, 1996). D-galactose was converted into galactitol, which is not metabolized by above enzymes but accumulate in the cell, that leads to osmotic stress and ROS production21. In addition, supplementation with antioxidants has been reported to be beneficial with respect to slowing the aging process22, 23.
Nan-Chai-Hu (Chai Hu of the South), the root of Bupleurum scorzonerifolium, is an important Chinese herb24. Isochaihulactone (also known as K8) is a lignan compound that was identified in acetone extracts of Nan-Chai-Hu and shows antitumor activity against A549 cells in vitro and in vivo25, 26. Lignan compounds (eg, sesamin, sesamolin) have been reported to act as neuroprotective agents against oxidative injury and excitotoxicity27, 28, 29, 30. Lignans can also inhibit lipopolysaccharide-inducible COX-2 expression in macrophages31. The aim of the present study was to investigate the effects of isochaihulactone on H2O2-induced injury in neuronal PC12 cells (nPC12) and in a murine D-gal–induced aging model.
Materials and methods
Fraction purification of isochaihulactone and structure determination
B scorzonerifolium roots were supplied from Chung-Yuan Co, Taipei, and the plant was identified by Professor Lin of the National Defense Medicinal Center, where a voucher specimen was deposited (NDMCP No 900801). The acetone extract AE-BS was prepared as described previously25, 32. The AE-BS was dissolved in 95% MeOH solution and then partitioned (1:1) with n-hexane to give the n-hexane-soluble fraction (BS-HE). The aqueous 95% MeOH layer was evaporated to remove residual MeOH, and then distilled water was added. This aqueous solution was further partitioned with CHCl3 to get the CHCl3-soluble fraction (BS-CE) and H2O-soluble fraction (BS-WE). The BS-CE was subjected to chromatography over silica gel and eluted with CH2Cl2, CH2Cl2-MeOH (95:5), CH2Cl2-MeOH (9:1), CH2Cl2-MeOH (8:2) and MeOH, successively. The bioactive component, BS-CE-E02, was applied to silica gel and eluted with CH2Cl2-MeOH (99:1) to obtain isochaihulactone. The pure compound, isochaihulactone forms white needle crystals with a physical properties of mp 137–138 8C; [a]D 25_29.08 (ca 0.5, CHCl3); IR (KBr) nmax cm_1: 1745, 1635, 1581, 1335, 1153; UV (CHCl3) lmax nm (log e): 247 (4.08), 298 (4.17), 327 (4.08); 1H NMR (CDCl3) d: 3.29 (1H, m, H-3), 4.10 (1H, dd, J=9.0, 3.8 Hz, H-4a), 4.31 (1H, dd, J=9.0, 7.3 Hz, H-4b), 6.60 (1H, d, J=1.5 Hz, H-5), 2.78 (1H, dd, J=13.7, 9.0 Hz, H-6a), 2.92 (1H, dd, J=13.7, 6.7 Hz, H-6b), 7.24s (2H, s, H-20, 60), 6.67 (1H, d, J=1.4 Hz, H-200), 6.74 (1H, d, J=7.8 Hz, H-500), 6.61 (1H, dd, J=7.8, 1.4 Hz, H-600), 3.87 (9H, s, OMe), 5.93 (1H, d, J=1.3 Hz, OCH2O), 5.94 (1H, d, J=1.3 Hz, OCH2O); 13C NMR (CDCl3) d: 169.29s (C-1), 126.36s (C-2), 44.43d (C-3), 69.82t (C-4), 140.60d (C-5), 40.72t (C-6), 128.83s (C-10), 108.65d (C-20), 152.62s (C-30), 139.61s (C-40), 152.62s (C-50), 108.65d (C-60), 131.31s (C-100), 109.29d (C-200), 147.94s (C-300), 146.49s (C-400), 108.39d (C-500), 122.29d (C-600), 56.18q (20-OMe), 60.90q (30-OMe), 56.18q (40-OMe), 101.03t (OCH2O); EIMS, 70 eV, m/z (rel int): 398 ([M]+, 18), 263 (100), 207 (16), 135 (35).
Chemicals and reagents
Isochaihulactone was dissolved in DMSO to a concentration of 100 mmol/L and stored in -20 °C as a stock solution. Dimethyl sulfoxide (DMSO), 3-(4,5-dimethyl thizol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), 2',7'-dichlorofluorescein diacetate (H2DCF-DA), Hoechst 33342, thiobarbituric acid (TBA), hydrogen peroxide (H2O2), trichloroacetic acid (TCA), malondialdehyde (MDA), propidium iodine (PI) and actin antibody were purchased from Sigma Chemical Co (St Louis, MO, USA). F-12 medium, horse serum, fetal bovine serum (FBS), penicillin, streptomycin, trypsin/EDTA and NuPAGE Bis-Tris Electrophoresis System (pre-cast polyacrylamide mini-gel) were purchased from Invitrogen (Carlsbad, CA, USA). CellBIND surface dishes and mouse 2.5S nerve growth factor (NGF) were purchased from Millipore (Bedford, MA). COX-2 antibody was purchased from Thermo scientific (Waltham, MA, USA). PARP antibodies and horseradish peroxidase- conjugated anti-mouse or anti-rabbit IgG secondary antibodies were purchased from Cell signaling (MA, USA). Polyvinyldifluoride (PVDF) membranes, BSA protein assay kit and Western blot chemiluminescence reagent were purchased from Amersham Biosciences (Arlington Heights, IL). Superoxide dismutase activity assay kit was purchased from BioVision (Mountain View, CA). Glutathione peroxidase assay kit was purchased from Cayman Chemical (MI, USA). DNA Fragmentation Assay Kit was purchased from Clontech Laboratories (Mountain View, CA). Non-Radioactive Cytoxicity Assay Kit was purchased from promega (Madison, WI, USA)
Cell culture and differentiation of neuronal PC12 cells
Undifferentiated rat phenchromocytoma cells (PC12 cells) were obtained from the Bioresources Collection and Research Center (BCRC, Hsin Chu, Taiwan) and maintained in F-12 medium supplemented with 2.5% fetal bovine serum and 12.5% horse serum in a CO2 incubator at 37 °C. To induce neuronal differentiation, PC12 cells grown on CellBIND surface dishes were incubated in the presence of 50 ng/mL of mouse 2.5S nerve growth factor (NGF). Experiments were carried out 72 h after NGF incubation while the percentage of neurite-bearing cells was added up to 80%−90%.
Establishment of D-galactose aging animal model
Male adult C57BL/6 mice were purchased from National Sciences Council (Taipei, Taiwan) weighing 28−30 g at the beginning of the experiment were used. Animals were randomly divided into three groups (control, D-gal-administration, and D-gal-administration plus isochaihulactone 10 mg/kg treatment) and maintained at 20 °C, 12 h light/12 h dark cycle with free access to food and water. D-Gal (100 mg/kg) was injected subcutaneously (sc) daily into mice for 6 weeks. isochaihulactone (10 mg/kg body weight) was injected subcutaneously (sc) 3 h prior to D-Gal injection. All control animals were given saline. The plasma of each group were collected for MDA content, antioxidative enzyme activity analysis.
Growth inhibition assay
Cell viability was assessed by measuring formazan produced by the reduction of MTT. Neuronal PC12 cells in 96-well plates were treated with H2O2 and incubated for 24 h at 37 °C. Isochaihulactone was added 3 h to the culture prior to H2O2 addition. The cells in each well were then incubated in culture medium with 500 mg/mL MTT for 2 h. Absorbance at 570 nm of the maximum was detected by a Spectramax Microplate ELISA Reader (Molecular Devices Corp, Sunnyvale, CA).
Cytotoxicity analysis
Lactate Dehydrogenase (LDH) Release Assay is used to measure cell membrane damage as a function of the amount of cytoplasmic LDH released into the medium. The LDH assay is based on the reduction of NAD+ by the action of LDH. The generated NADH is utilized for stoichiometric conversion of tetrazolium dye. LDH activity can be used as an indicator of cytotoxicity. Neuronal PC12 cells in 96-well plates were treated with H2O2 and incubated for 24 h at 37 °C. The 100 μmol/L α-tocopherol was used as a positive control (PC). Isochaihulactone or 100 μmol/L α-tocopherol was added 3 h to the culture prior to H2O2 addition, and LDH content was determined using the Non-Radioactive Cytoxicity Assay (Promega). The test was performed according to the manufacturer's protocol. Briefly, at the end of the incubation, an aliquot of the medium (50 μL) was added to the kit reagent and incubated for 30 min, and then the reaction was stopped and the absorbance was measured at 490 nm using a microplate reader.
In situ TdT-mediated dUTP nick end labeling (TUNEL) assay
Apoptotic cells were confirmed with the DNA Fragmentation Assay Kit (clontech), in accordance with the manufacturer's instructions. Neuronal PC12 cells in 96-well plates were treated with H2O2 and incubated for 24 h at 37 °C. Isochaihulactone was added 3 h to the culture prior to H2O2 addition, then cells were fixed in 4% paraformaldehyde for 25 min at 4 °C, and then permiabilized with 0.2% Triton X-100 for 5 min at room temperature. Free 3′ ends of fragmented DNA were enzymatically labeled with the TdT-mediated dUTP nick end labeling (TUNEL) reaction mixture for 60 min at 37 °C in a humidified chamber. Cell nuclei was monitored by Propidium Iodine (PI) staining. Labeled DNA fragments were monitored by fluorescence microscopy (Zeiss).
Hoechst 33342 staining
After a 24 h treatment of the cells with H2O2 (200 μmol/L), Hoechst 33248 staining was performed. Isochaihulactone was added 3 h prior to H2O2 stimulation. Neuronal PC12 cells were stained with Hoechst 33248 dye to evaluate apoptosis. The cells were fixed with 4% paraformadehyde at room temperature and stained with Hoechst 33342 working solution (5 μmol/L) for 30 min, then washed with PBS. Fluorescence was visualized using a fluorescent microscope (Zeiss) under 200× magnification.
Intracellular reactive oxygen species detection
The production of intracellular reactive oxygen species was estimated by using a fluorescent probe, 2',7'-dichlorofluorescein diacetate (DCFH-DA). DCFH-DA is transported across the cell membrane and hydrolyzed by intracellular esterases to form non-fluorescent 2',7'-dichlorofluorescein (DCFH), which is then rapidly converted to highly fluorescent 2',7'-dichlorofluorescin (DCF) in the presence of reactive oxygen species. The DCF fluorescence intensity is believed to be parallel to the amount of reative oxygen species formed intracellularly. After 24 h treatment with 200 μmol/L H2O2, Cells were collected and CH2 DCFDA was added (final concentration 10 μmol/L) for 60 min at 37 °C. Cells were washed by PBS for at least three times. The production of reactive oxygen species was measured immediately by Cell lab QuantaTMSC Flow cytometer (Beckman coulter).
Measurement of MDA content and antioxidant enzyme activities
The content of MDA was determined using the thiobarbituric acid method. Equal volumes of 0.67 % thiobarbituric acid reagent was added to the sample supernatant and boiled for 10 min at 100 °C, and cooled, the absorbance of each supernatant was measured at 532 nm. MDA content was calculated by MDA standard. Antioxidant enzyme activities were assayed with Superoxide dismutase activity assay kit (BioVision) and glutathione peroxidase assay kit (Cayman). The assay was in accordance with the manufacturer's instructions.
RNA extraction and RT-PCR assay
Total RNA from each sample was isolated by RNeasy (Qiagen, Valencia, CA, USA), according to the manufacturer's specifications. RNA quality was assessed using agarose gel electrophoresis. The concentration was calculated spectophotometrically and 1 μg of total-RNA from each sample was used to generate cDNA using the Omniscript RT kit (Qiagen) according to manufacturer's protocol. One micrograms of cDNA was amplified in the presence of 1 mmol/L primers: cox2: (F) 5′-ACACTCTATCACTGGCATCC-3′ and (R) 5′-GAAGGGACACCCTTTCACAT-3′, cox1: (F) 5′-TTTGCACA ACACTTCACCCACCAG-3′ and (R) 5′-AAACACCTCCTGGCCCACAGCCAT-3′, p50: (F) 5′-GTCTCAAACCAAACAGCCTCAC-3′ and (R) 5′-CAGTGTCTTCCTCGACATGGAT-3′, rela: (F) 5′-GTCTCAAACCAAACAGCCTCAC-3′ and (R) 5′-CAGTGTCTTCCTCGACATGGAT-3′, sod1: (F) 5′-AAGGCCGTGTGCGTGCTGAA-3′ and (R) 5′-CAGGTCTCCAACATG CCTCT-3′, sod2: (F) 5′-CAGAGGCACAATGTCACTCCTC-3′ and (R) 5′-TTTATGGCCACAGTTTCACAGAA-3′and gapdh: (F) 5′-TGAAGGTCG GAGTCAACGGATTTGGT-3′ and (R) 5′-CATGTGGGCCATGAGGTCCACCAC-3′, with Taq DNA polymerase. The thermal cycling profile was composed of an initial denaturation step at 95 °C for 10 min, 30 cycles of 30 s of denaturation at 95 °C, 30 s of annealing at 58 °C (cox1, cox2, and gapdh) or 52 °C (sod1, sod2, rela, and p50) , and 1 min of extension at 72 °C, with a final 5 min extension step at 72 °C. The intensity of bands was analyzed by AC Imaging System (LS Image Acquisition Software, UVP).
Western blot analysis
Neuronal PC12 cells in 96-well plates were treated with H2O2 and incubated for 24 h at 37 °C. Isochaihulactone or 100 μmol/L α-tocopherol (PC) was added 3 h to the culture prior to H2O2 addition, The cells were lysed on ice with 200 mL of lysis buffer (50 mmol/L Tris–HCl, pH 7.5, 0.5 mol/L NaCl, 5mmol/L MgCl2, 0.5% nonidet P-40, 1 mmol/L phenylmethylsulfonyl fluoride, 1 mg/mL pepstatin, and 50 mg/mL leupeptin) and centrifuged at 13 000×g at 4 °C for 20 min. The protein concentrations in the supernatants were quantified using a BSA Protein Assay Kit. Electrophoresis was performed on a NuPAGE Bis–Tris Electrophoresis System using 50 mg of reduced protein extract per lane. Resolved proteins were then transferred to polyvinyldifluoride (PVDF) membranes. Filters were blocked with 5% non-fat milk overnight and probed with appropriate dilution of primary antibodies for 1 h at room temperature. Membranes were washed with three times with 0.1% Tween 20 and incubated with HRP-conjugated secondary antibody for 1 h at room temperature. All proteins were detected using Western Lightning™ Chemiluminescence Reagent Plus and quantified using a densitometers.
Statistical analysis
The data represent means±SD. Statistical differences were analyzed using the Student's t-test. For the pairwise comparisons multiple samples, statistical differences were analyzed using the t-test to compare the specific pairs of groups in one-way ANOVA (LSD procedure). Values of P<0.05 were considered significant.
Results
Isochaihulactone protected nPC12 cells against H2O2-induced cytotoxicity and apoptosis
The viability of nPC12 cells in response to exposure to 200 μmol/L H2O2 for 24 h was significantly (P<0.05) decreased, to 71% of that of control cells. Cells were also pretreated with isochaihulactone (Figure 1) or 100 μmol/L α-tocopherol (a potent antioxidant) 3 h before the addition of H2O233. Pretreatment with isochaihulactone (5 μmol/L or 10 μmol/L) significantly (P<0.05) inhibited this decrease (Figure 2A), whereas 40 μmol/L isochaihulactone did not exert any protective effect. To assess membrane damage, cells were treated with isochaihulactone (5 μmol/L or 10 μmol/L), and H2O2-induced cytotoxicity was determined by LDH assay. Treatment with H2O2 for 24 h showed an increase in LDH release compared to the control group, to 53.2%. Pretreatment with isochaihulactone (5 μmol/L or 10 μmol/L) significantly decreased LDH release, from 54.1% (vehicle-treated group) to 35.5% (5 μmol/L) and 27.6% (10 μmol/L). Pretreatment with 100 μmol/L α-tocopherol also significantly attenuated this increase in LDH release. There was no significant difference between the effect of isochaihulactone and that of α-tocopherol (Figure 2B).
Attenuation of H2O2-induced injury cell by isochaihulactone in neuronally differentiated PC12 cells (nPC12). Isochaihulactone or 100 μmol/L α-tocopherol was added to the cultures 3 h before the addition of H2O2. Cells were incubated with 200 μmol/L H2O2 for 24 h for MTT, LDH or apoptosis assay. Pretreatment with isochaihulactone protected nPC12 cells against H2O2-induced injury by increasing cell viability (A) and decreasing H2O2-induced cytotoxicity. The 100 μmol/L α-tocopherol was used as a positive control (PC). (B) In addition, isochaihulactone (10 μmol/L) pretreatment decreased DNA fragmentation (C), chromatin condensation (D) Caspase-3 and PARP cleavage (E), apoptotic characteristics induced by H2O2. Data are presented as mean±standard deviation (SD) (n=3). bP<0.05 as compared to control group; eP<0.05 as compared to H2O2 treated group.
We also assessed apoptosis in nPC12 cells by TUNEL assay, morphologic analysis of cell nuclei and poly (ADP-ribose) polymerase (PARP) degradation. Treatment of cells with H2O2 induced apoptosis, which was inhibited by pretreatment with isochaihulactone. In vehicle-treated control groups, cells were negative for TUNEL fluorescence. After exposure to 200 μmol/L H2O2 for 24 h, the percentage of TUNEL-positive cell increased. Pretreatment with isochaihulactone (10 μmol/L) for 3 h decreased the percentage of TUNEL-positive cells and significantly reduced apoptosis level back to control (Figure 2C). We next evaluated apoptosis via Hoechst 33342 staining to assess changes in nuclear morphology. As shown in Figure 2D, pretreatment with isochaihulactone (10 μmol/L) decreased the amount of chromatin condensation induced by H2O2. In addition, pretreatment with isochaihulactone for 3 h significantly (P<0.05) inhibited the H2O2-induced increase in caspase-3 and PARP activation (Figure 2E).
Isochaihulactone increased the antioxidant response of nPC12 cells
We assessed the level of intracellular ROS by DCFH-DA assay. Treatment of nPC12 cells with 200 μmol/L H2O2 for 24 h resulted in a 1.61-fold increase in intracellular ROS compared to vehicle-treated control cells. Coincubation with isochaihulactone (5 μmol/L or 10 μmol/L) significantly decreased ROS production compared to that in the vehicle-treated group (Figure 3A). Treatment with H2O2 markedly increased the level of the lipid peroxidation product MDA (Figure 3B) and decreased the antioxidant enzymatic activities of SOD and GPx (Figure 3C, 3D). Pretreatment with isochaihulactone (5 μmol/L or 10 μmol/L) resulted in a noticeable decrease in the MDA level and increased SOD and GPx activities compared to those in the vehicle-treated group. In addition, SOD and GPx activities in nPC12 cells treated with isochaihulactone (5 μmol/L or 10 μmol/L) for 24 h showed no significant difference compared to control cells. Expression of SOD1 and SOD2 mRNA was downregulated in nPC12 cells in response to treatment with H2O2. Pretreatment with isochaihulactone inhibited this effect (Figure 3E), indicating that isochaihulactone not only elevated the activity of these antioxidant enzymes but also attenuated the decrease in SOD1 and SOD2 expression induced by H2O2.
Effect of isochaihulactone on H2O2-induced intracellular accumulation of reactive oxygen species (ROS) and lipid peroxidation and downregulation of antioxidant enzyme (SOD and GPx) activity in neuronally differentiated PC12 cells (nPC12). Pretreatment with isochaihulactone attenuated the H2O2-induced accumulation of ROS (A) and lipid peroxidation (B). In addition, isochaihulactone (10 μmol/L) pretreatment maintained the activity of SOD (C) and GPx (D) as controls. Isochaihulactone also rescued mRNA transcription of SOD1 and SOD2, which was inhibited by H2O2 (E). Data are presented as mean ± standard deviation (SD) (n=3). bP<0.05 as compared to control group; eP<0.05 as compared to H2O2 treated group.
Isochaihulactone inhibited COX-2 expression in H2O2-treated nPC12 cells
Reactive oxygen species can themselves increase cellular COX-2 expression. By Western blot analysis, pretreatment with isochaihulactone blocked H2O2-induced COX-2 mRNA and protein expression in nPC12 cells but had no effect on COX-1 mRNA expression (Figure 4A, 4B). The transcription factor NF-kappa B is important in the regulation of COX-2 expression. Therefore, NF-kappa B mRNA expression was assessed after incubation of nPC12 cells with 200 μmol/L H2O2 for 3 h. mRNA expression of the NF-kappa B subunits P50 and RELA was downregulated by pretreatment with isochaihulactone (Figure 4C), indicating that isochaihulactone decreased the expression of COX-2 via downregulation of NF-kappa B.
Modulation of the cyclooxygenase 2 (COX-2) isozyme and NF-kappa B subunits (RELA and P50) by isochaihulactone pretreatment in H2O2-treated neuronal PC12 cells (nPC12). Treatment with H2O2 induced mRNA expression of COX-2, but not of COX-1, and isochaihulactone pretreatment decreased this mRNA increase (A). Isochaihulactone pretreatment also decreased COX-2 protein expression induced by H2O2 (B). In addition, pretreatment with isochaihulactone decreased the mRNA expression of RELA and P50 (C). Data are presented as mean±standard deviation (SD) (n=3). Relation to control in (A) to (C) is relative to untreated control group.
Antioxidant effects of isochaihulactone in the D-galactose aging model
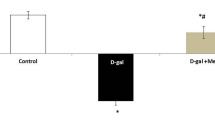
We measured the activities of T-SOD and GSH-Px and the MDA level in the plasma of mice. The MDA level in D-gal–treated mice was significantly increased compared to that in the control group (Figure 5A–5C). Administration of isochaihulactone (10 mg·kg-1·d-1) significantly inhibited this increase. The activities of T-SOD and GSH-Px in D-gal–treated mice were significantly decreased compared to those in the control group, and administration of isochaihulactone (10 mg·kg-1·d-1) significantly attenuated these decreases. Furthermore, we used H&E staining to quantify cell death within hippocampus: counting of pyknotic nuclei in H&E section. The result showed that percentage of pyknotic nuclei in the D-gal-treated mice were much higher than in control. Animals that received isochaihulactone showed a significantly decrease in the percentage of the damaged cells with respect to D-gal-receiving mice (Figure 5D).
Effect of isochaihulactone on plasma MDA level and SOD and GPx activities in D-galactose-treated (aged) mice. The control group received subcutaneous (sc) injections of phosphate-buffered saline. The aged group received D-galactose (100 mg/kg, sc). The isochaihulactone group received D-galactose (100 mg·kg-1·d-1, sc) plus isochaihulactone (10 mg·kg-1·d-1, sc). Treatments were administered for 7 weeks. Isochaihulactone treatment attenuated the aging characteristics of increased MDA level and downregulated SOD and GPx activities. In addition, neuronal damage analysis. H&E staining shows that pyknotic nucleis in galactose-treated group (middle) were significantly increased compared with vehicle-treated group (left) and decreased in galactose+isochaihulactone treated group (right) compared with galactose alone group in the CA1 subfield of hippocampus after 7 weeks of administration (D). Data are presented as mean±standard deviation (SD) (n=3 mice). bP<0.05 as compared to control group; eP<0.05 as compared to H2O2 treated group.
Discussion
Results of the present study provide evidence that isochaihulactone can exert neuroprotective effects against H2O2-induced oxidative stress in nPC12 cells. Pretreatment with isochaihulactone inhibited intracellular ROS formation. Although a small proportion of H2O2 may be scavenged by cellular antioxidant enzymes, it nonetheless directly induces the oxidation of various intracellular targets including the fluorescence probe DCFH-DA. When cells were exposed to exogenous H2O2, DCF fluorescence increased significantly. The formation of hydroxyl radicals mediated by intracellular heavy metal ions may also contribute to the increased DCF fluorescence in response to H2O2. Many reports indicate that lignans can access intracellular locations, owing to their benzylic structures, justifying their ability to attenuate oxidative stress induced by diverse stimuli34, 35. The chemical structure of isochaihulactone (sugar moiety attached to the 20 position of the triterpene dammarane) may contribute to its direct antioxidant properties36. However, antioxidant activity was also found in other cellular models, and the concentrations of isochaihulactone required for neuroprotection were far lower than those of H2O2 used in our present experiments, suggesting that it may not be a simple stoichiometric interaction.
Antioxidant activity of isochaihulactone was observed in the present study at concentrations of 5 μmol/L and 10 μmol/L, whereas 40 μmol/L isochaihulactone showed no protective effects. In our previous study, we found isochaihulactone caused cytotoxicity in various cancer cell lines including lung, breast, ovary, colon, liver tumor cells (IC50=10–50 μmol/L after 48h), paclitaxel-resistant A549-T12 and P-gp-overexpression KB-TAX50 cells25. In this study, we found that antioxidant activity of isochaihulactone was observed at concentrations of 5 μmol/L and 10 μmol/L. These results revealed that isochaihulactone may activate different pathway through different concentration and cell types. Consistently, it has been reported that a major mammalian metabolite of plant-based liganans enterolactone act as antioxidants at relatively low concentrations with maximum protection at 100 μmol/L37 and also used to induce anticancer activity of prostate cancer at higher than 100 μmol/L38. In addition, the PC12 cells used in the present study are clonal cells derived from rat pheochromocytoma. Treatment with nerve growth factor induces the differentiation of PC12 cells into a sympathetic neuron-like phenotype39. This cell line has been used widely as a model in neurobiologic, neuropharmacologic and neurotoxicologic studies. The response of PC12 cells to isochaihulactone may not be exactly the same as that observed in other cells. Therefore, isochaihulactone exerts potent antiaging effects against D-gal in mice via antioxidative mechanisms at low dosage but a strong anti-proliferative effect at high dosage.
The cyclooxygenase (COX) enzymes catalyze a key step in the conversion of arachidonate to PGH2, the immediate substrate for a series of cell specific prostaglandin and thromboxane synthases. There are two COX isoforms, which differ mainly in their pattern of expression. COX-1 is expressed in most tissues, whereas COX-2 usually is absent, but is induced by numerous physiologic stimuli. Results of the present study showed that isochaihulactone inhibited the expression of COX-2 and decreased lipid peroxidation. The dual intrinsic enzyme activities of COX-2 catalyze two sequential reactions in the metabolism of arachidonic acid (AA). The COX-2 enzyme possesses cyclooxygenase activity that metabolizes AA to hydroperoxide (PGG2; 9,11-endo-peroxy-15-hydroperoxyprostaglandin) utilizing two oxygen molecules (2O2), and it also possesses a heme-containing active site that provides peroxidase activity, which requires two electrons (2e−) to become active. The peroxidase reaction converts PGG2 to PGH2 by removing oxygen(s), [Ox], which may be a source of oxygen radicals. Therefore, as more AA is metabolized to PG by COX-2, more electron donors are depleted, and more oxygen radicals are generated. The COX-2–dependent production of ROS is likely to be involved in the enhanced lipid peroxidation in H2O2-treated cells. The mechanism for the induction of COX-2 in H2O2-induced apoptosis of nPC12 cells is unknown. The COX-2 inhibitor U0126 blocks hypoxia-induced MAPK/ERK1/2 activity in PC12 cells after 1 h of hypoxia and significantly protects against hypoxic death40, suggesting that COX-2 activation is involved in hypoxia in PC12 cells. Results of the present study showed that H2O2 increased the expression of COX-2 and the transcription factor p65 in nPC12 cells and that pretreatment with isochaihulactone inhibited this effect and decreased the level of LDH release in response to H2O2 treatment. This result indicates that isochaihulactone may also regulate MAPK signaling to protect nPC12 cells against H2O2-induced injury.
Many studies have shown that lignans possess potent antioxidant properties in vitro and in vivo. There have been no previous reports on the protective effect of isochaihulactone against D-gal–induced aging in mice. To protect cells against oxidative damage induced by ROS, the antioxidant system in the body is activated, and endogenous antioxidant enzymes, such as SOD and GPx, scavenge ROS or prevent their formation. The production of ROS can also be evaluated indirectly by analyzing the level of MDA, a product of free radical-induced lipid peroxidation. Analysis of the number of pyknotic nuclei cells in the hippocampus showed that isochaihulactone had an important protective effect against D-gal-induced cell death. Overall, our present findings suggest that isochaihulactone can protect mice against oxidative stress injury induced by D-gal and improves impairments in aging mice.
In conclusion, isochaihulactone decreased oxidative stress-induced ROS production and lipid peroxidation and also maintained endogenous antioxidant enzymatic activities, stabilized mitochondrial function, and subsequently attenuated nPC12 cell injury. Although more detailed mechanistic studies are necessary to clarify the mechanism of neuroprotection by isochaihulactone, these results should encourage further studies to explore the potential neuroprotective effects of isochaihulactone in neurologic diseases.
Author contribution
Yi-lin Sophia CHEN designed research; Sung-liang YU, Shih-bin LIN, Yung-luen YU, Min-hui CHIEN, Kuo-jung SU, Ching-ju LIN performed research; Tzong-der WAY, Giou-teng YIANG contributed new analytical tools and reagents; Chai-ching LIN, De-chuan CHAN, Horng-jyh HARN analyzed data; Sung-liang YU wrote the paper
References
Barnham KJ, Masters CL, Bush AI . Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov 2004; 3: 205–14.
Finkel T, Holbrook NJ . Oxidants, oxidative stress and the biology of ageing. Nature 2000; 408: 239–47.
Yin ST, Tang ML, Deng HM, Xing TR, Chen JT, Wang HL, et al. Epigallocatechin-3-gallate induced primary cultures of rat hippocampal neurons death linked to calcium overload and oxidative stress. Naunyn Schmiedebergs Arch Pharmacol 2009; 379: 551–64.
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J . Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007; 39: 44–84.
Floyd RA, Soong LM, Stuart MA, Reigh DL . Free radicals and carcinogenesis. Some properties of the nitroxyl free radicals produced by covalent binding of 2-nitrosofluorene to unsaturated lipids of membranes. Arch Biochem Biophys 1978; 185: 450–7.
Tachon P . DNA single strand breakage by H2O2 and ferric or cupric ions: its modulation by histidine. Free Radic Res Commun 1990; 9: 39–47.
Willson RL . Peroxy free radicals and enzyme inactivation in radiation injury and oxygen toxicity: protection by superoxide dismutase and antioxidants? Lancet 1984; 1: 804.
Nakamura T, Sakamoto K . Reactive oxygen species up-regulates cyclooxygenase-2, p53, and Bax mRNA expression in bovine luteal cells. Biochem Biophys Res Commun 2001; 284: 203–10.
Adderley SR, Fitzgerald DJ . Oxidative damage of cardiomyocytes is limited by extracellular regulated kinases 1/2-mediated induction of cyclooxygenase-2. J Biol Chem 1999; 274: 5038–46.
Feng L, Xia Y, Garcia GE, Hwang D, Wilson CB . Involvement of reactive oxygen intermediates in cyclooxygenase-2 expression induced by interleukin-1, tumor necrosis factor-alpha, and lipopolysaccharide. J Clin Invest 1995; 95: 1669–75.
Lee AK, Sung SH, Kim YC, Kim SG . Inhibition of lipopolysaccharide-inducible nitric oxide synthase, TNF-alpha and COX-2 expression by sauchinone effects on I-kappaBalpha phosphorylation, C/EBP and AP-1 activation. Br J Pharmacol 2003; 139: 11–20.
Li L, Prabhakaran K, Shou Y, Borowitz JL, Isom GE . Oxidative stress and cyclooxygenase-2 induction mediate cyanide-induced apoptosis of cortical cells. Toxicol Appl Pharmacol 2002; 185: 55–63.
Zhang ZF, Fan SH, Zheng YL, Lu J, Wu DM, Shan Q, et al. Purple sweet potato color attenuates oxidative stress and inflammatory response induced by D-galactose in mouse liver. Food Chem Toxicol 2009; 47: 496–501.
Cui X, Wang L, Zuo P, Han Z, Fang Z, Li W, et al. D-galactose-caused life shortening in Drosophila melanogaster and Musca domestica is associated with oxidative stress. Biogerontology 2004; 5: 317–25.
Fang F, Liu G . A novel cyclic squamosamide analogue compound FLZ improves memory impairment in artificial senescence mice induced by chronic injection of D-galactose and NaNO2 . Basic Clin Pharmacol Toxicol 2007; 101; 447–54.
He M, Zhao L, Wei MJ, Yao WF, Zhao HS, Chen FJ . Neuroprotective effects of (–)-epigallocatechin-3-gallate on aging mice induced by D-galactose. Biol Pharm Bull 2009; 32: 55–60.
Jordens RG, Berry MD, Gillott C, Boulton AA . Prolongation of life in an experimental model of aging in Drosophila melanogaster. Neurochem Res 1999; 24: 227–33.
Wei H, Li L, Song Q, Ai H, Chu J, Li W . Behavioural study of the D-galactose induced aging model in C57BL/6J mice. Behav Brain Res 2005; 157: 245–51.
Xu XH, Zhang ZG . Effect of puerarin on learning-memory behavior and synaptic structure of hippocampus in the aging mice induced by D-galactose. Yao Xue Xue Bao 2002; 37: 1–4.
Shen YX, Xu SY, Wei W, Sun XX, Yang J, Liu LH, et al. Melatonin reduces memory changes and neural oxidative damage in mice treated with D-galactose. J Pineal Res 2000; 32: 173–8.
Kumar A, Prakash A, Dogra S . Naringin alleviates cognitive impairment, mitochondrial dysfunction and oxidative stress induced by D-galactose in mice. Food Chem Toxicol 2010; 48: 626–32.
Sack CA, Socci DJ, Crandall BM, Arendash GW . Antioxidant treatment with phenyl-alpha-tert-butyl nitrone (PBN) improves the cognitive performance and survival of aging rats. Neurosci Lett 1996; 205: 181–4.
Cui X, Zuo P, Zhang Q, Li X, Hu Y, Long J, et al. Chronic systemic D-galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice: protective effects of R-alpha-lipoic acid. J Neurosci Res 2006; 84: 647–54.
Liu JH, Ho SC, Lai TH, Liu TH, Chi PY, Wu RY . Protective effects of Chinese herbs on D-galactose-induced oxidative damage. Methods Find Exp Clin Pharmacol 2003; 25, 447–52.
Chen YL, Lin SZ, Chang JY, Cheng YL, Tsai NM, Chen SP, et al. In vitro and in vivo studies of a novel potential anticancer agent of isochaihulactone on human lung cancer A549 cells. Biochem Pharmacol 2006; 72: 308–19.
Chen YL, Lin PC, Chen SP, Lin CC, Tsai NM, Cheng YL, et al. Activation of nonsteroidal anti-inflammatory drug-activated gene-1 via extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase revealed a isochaihulactone-triggered apoptotic pathway in human lung cancer A549 cells. J Pharmacol Exp Ther 2007; 323: 746–56.
Hou RC, Huang HM, Tzen JT, Jeng KC . Protective effects of sesamin and sesamolin on hypoxic neuronal and PC12 cells. J Neurosci Res 2003; 74: 123–33.
Hamada N, Fujita Y, Tanaka A, Naoi M, Nozawa Y, Ono Y, et al. Metabolites of sesamin, a major lignan in sesame seeds, induce neuronal differentiation in PC12 cells through activation of ERK1/2 signaling pathway. J Neural Transm 2009; 116: 841–52.
Jang YP, Kim SR, Choi YH, Kim J, Kim SG, Markelonis GJ, et al. Arctigenin protects cultured cortical neurons from glutamate-induced neurodegeneration by binding to kainate receptor. J Neurosci Res 2002; 68: 233–40.
Yang XW, He HP, Du ZZ, Liu HY, Di YT, Ma YL, et al. Tarennanosides A-H, eight new lignan glucosides from Tarenna attenuata and their protective effect on H2O2-induced impairment in PC12 cells. Chem Biodivers 2009; 6: 540–50.
Yun KJ, Min BS, Kim JY, Lee KT . Styraxoside A isolated from the stem bark of Styrax japonica inhibits lipopolysaccharide-induced expression of inducible nitric oxide synthase and cyclooxygenase-2 in RAW 264.7 cells by suppressing nuclear factor-kappa B activation. Biol Pharm Bull 2007; 30: 139–44.
Chang WL, Chiu LW, Lai JH, Lin HC . Immunosuppressive flavones and lignans from Bupleurum scorzonerifolium. Phytochemistry 2003; 64: 1375–9.
Tome Ada R, Ferreira PM, de Freitas RM . Inhibitory action of antioxidants (ascorbic acid or alpha-tocopherol) on seizures and brain damage induced by pilocarpine in rats. Arq Neuropsiquiatr 2010; 68: 355–61.
Yamauchi S, Hayashi Y, Nakashima Y, Kirikihira T, Yamada K, Masuda T . Effect of benzylic oxygen on the antioxidant activity of phenolic lignans. J Nat Prod 2005; 68: 1459–70.
Yamauchi S, Sugahara T, Matsugi J, Someya T, Masuda T, Kishida T, et al. Effect of the benzylic structure of lignan on antioxidant activity. Biosci Biotechnol Biochem 2007; 71: 2283–90.
Sridhar C, Rao KV, Subbaraju GV . Flavonoids, triterpenoids and a lignan from Vitex altissima. Phytochemistry 2005; 66: 1707–12.
Kitts DD, Yuan YV, Wijewickreme AN, Thompson LU . Antioxidant activity of the flaxseed lignan secoisolariciresinol diglycoside and its mammalian lignan metabolites enterodiol and enterolactone. Mol Cell Biochem 1999; 202: 91–100.
Chen LH, Fang J, Li H, Demark-Wahnefried W, Lin X . Enterolactone induces apoptosis in human prostate carcinoma LNCaP cells via a mitochondrial-mediated, caspase-dependent pathway. Mol Cancer Ther 2007; 6: 2581–90.
Luckenbill-Edds L, Van Horn C, Greene LA . Fine structure of initial outgrowth of processes induced in a pheochromocytoma cell line (PC12) by nerve growth factor. J Neurocytol 1979; 8: 493–511.
Huang HM, Yu JY, Ou HC, Jeng KC . Effect of naloxone on the induction of immediately early genes following oxygen- and glucose-deprivation in PC12 cells. Neurosci Lett 2008; 438: 252–6.
Acknowledgements
This work was supported by grants from the National Science Council of Taiwan NSC962320-B-039-032-MY3 and NSC963111-B-039-003 to YLY, and NSC96-2320-B-039-044 -MY3 to HJH, and NSC98-2320-B-197-002-MY3 to YLC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, Sl., Lin, Sb., Yu, Yl. et al. Isochaihulactone protects PC12 cell against H2O2 induced oxidative stress and exerts the potent anti-aging effects in D-galactose aging mouse model. Acta Pharmacol Sin 31, 1532–1540 (2010). https://doi.org/10.1038/aps.2010.152
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2010.152
Keywords
This article is cited by
-
d-Galactose-induced accelerated aging model: an overview
Biogerontology (2019)