Abstract
Ulcerative colitis (UC) is a refractory inflammatory bowel disease, which is known to cause psychiatric disorders such as anxiety and depression at a high rate in addition to peripheral inflammatory symptoms. However, the pathogenesis of these psychiatric disorders remains mostly unknown. While prior research revealed that the Enterococcus faecalis 2001 (EF-2001) suppressed UC-like symptoms and accompanying depressive-like behaviors, observed in a UC model using dextran sulfate sodium (DSS), whether it has an anxiolytic effect remains unclear. Therefore, we examined whether EF-2001 attenuates DSS-induced anxiety-like behaviors. Treatment with 2% DSS for seven days induced UC-like symptoms and anxiety-like behavior through the hole-board test, increased serum lipopolysaccharide (LPS) and corticosterone concentration, and p-glucocorticoid receptor (GR) in the prefrontal cortex (PFC), and decreased N-methyl-d-aspartate receptor subunit (NR) 2A and NR2B expression levels in the PFC. Interestingly, these changes were reversed by EF-2001 administration. Further, EF-2001 administration enhanced CAMKII/CREB/BDNF-Drebrin pathways in the PFC of DSS-treated mice, and labeling of p-GR, p-CAMKII, and p-CREB showed colocalization with neurons. EF-2001 attenuated anxiety-like behavior by reducing serum LPS and corticosterone levels linked to the improvement of UC symptoms and by facilitating the CAMKII/CREB/BDNF-Drebrin pathways in the PFC. Our findings suggest a close relationship between UC and anxiety.
Similar content being viewed by others
Introduction
Ulcerative colitis (UC) is a refractory inflammatory bowel disease (IBD) that affects approximately 1.4 million people in the United States and 2.2 million people in Europe. Patients with IBD, including UC and Crohn’s disease, have been reported to develop psychiatric disorders such as obsessive–compulsive disorder, panic disorder, anxiety, depression, and autism at a high rate1,2,3,4,5; however, the mechanism of pathogenesis of these disorders remains unclear.
The composition of the intestinal microbiota in patients with IBD differs from that of healthy individuals, resulting in an imbalance between gram-positive and gram-negative bacteria6. Lipopolysaccharide (LPS), a component of the outer membrane of gram-negative bacteria, may be transferred into the bloodstream in IBD due to increased permeability of the intestinal cell membrane following enteritis symptoms6,7,8. In animal studies, intraperitoneal administration of LPS has been reported to act on the adrenal cortex and increase blood corticosterone levels, resulting in anxiety-like behavior9,10,11. Hyperactivation in the brain of the glucocorticoid receptor (GR), a receptor for corticosterone, is implicated in the development of anxiety-like behavior12,13,14,15. From these findings, it can be surmised that gut microbiota imbalance may adversely affect the brain through elevated serum LPS and corticosterone, leading to anxiety symptoms.
Activation of GR in the ventral hippocampus and prefrontal cortex (PFC) suppresses both the expression of N-methyl-d-aspartate receptor (NMDAR) subunit (NR) 2A and NR2B as well as anxiety-like behavior16,17,18,19,20,21,22. NMDAR is composed of five subunits (NR1, NR2A, NR2B, NR2C, and NR2D) and the absence of these subunits results in reduced phosphorylation levels of the calcium/calmodulin-dependent protein kinase (CAMK) II/cAMP-responsive element-binding protein (CREB) pathway23,24,25. Phosphorylation of CREB regulates the expression of brain-derived neurotrophic factor (BDNF) and drebrin, which are involved in synaptic plasticity; studies have reported these proteins to be implicated in anxiolytic effects23,26,27. These findings suggest that GR inactivation may have an anxiolytic effect by activating synaptic plasticity.
Dextran sulfate sodium (DSS), which causes inflammatory changes in the intestinal mucosa similar to UC, has shown to induce depression-like and anxiety-like behaviors28,29,30,31,32. Moreover, DSS-treated mice exhibit the disruption of gut microbiota balance, decreased intestinal barrier integrity, and elevated serum LPS, as well as elevated serum corticosterone (cortisol in humans) observed in patients with anxiety29,31,33,34,35. Therefore, we considered that this model may be useful in the study of psychiatric disorders associated with IBD.
Enterococcus faecalis 2001 (EF-2001) is a probiotic lactic acid bacterium used as a biological response modifier (BRM), produced by heat treating live E. faecalis 2001. EF-2001 is a unique strain of E. faecalis with no other strains identical to E. faecalis based on the Multilocus Sequence Typing analysis method; it has been reported to exhibit anti-inflammatory and anti-tumor effects through immune function modulation32,36,37,38,39,40. Previous studies demonstrated that EF-2001 reduced DSS-induced IBD-like symptoms and depression-like behaviors, as well as olfactory bulbectomy-induced depression-like behaviors and memory deficits32,41,42. However, the effect of EF-2001 on colitis-induced anxiety is unclear.
Against this background, and addressing the abovementioned gap in prior knowledge, we investigated whether EF-2001 attenuates DSS-induced changes in anxiety-like behavior and peripheral symptoms, and examined the molecular mechanisms underlying these effects.
Materials and methods
All experiments were approved by the Ethics Committee of Animal Experiments at the International University of Health and Welfare (Ohtawara, Japan; approval number: 19015, 22009). All animal experiments complied with the ARRIVE guidelines and were performed in accordance with the guidelines established by the Animal Research Committee of the International University of Health and Welfare and the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals. Efforts were made to minimize the suffering of the animals and reduce the number of animals used in the experiments. Measurements of all experiments and analyses were performed in a blinded manner.
Animals
We used male ddY mice (age, 6–7 weeks; weight, 26–28 g; Japan SLC, Shizuoka, Japan) for all the experiments (total: n = 281; behavioral tests: n = 221; western blot analysis: n = 30; enzyme-linked immunosorbent assay (ELISA) analysis: n = 24; immunohistochemical study: n = 6). The mice were housed in cages containing five to six animals, and subjected to steady conditions (i.e., temperature, 23 ± 1 °C; humidity, 55 ± 5%, and 12/12 h light–dark cycle with lights on at 7:00). All behavioral tests were performed between 10:00 and 17:00. Each animal was tested only once for each behavioral test. The behavioral tests were conducted by an observer blinded to other study information. Mice were euthanized by cervical dislocation by skilled personnel, except for mice from which samples were collected for western blot, ELISA, and immunohistochemical studies.
Drugs and treatments
Commercially available heat-treated EF-2001 was originally isolated from healthy human feces. It was supplied as a heat-killed, dried powder by Nihon Berm Co., Ltd. (Tokyo, Japan). DSS (1.5%, 2%, or 2.5%; Wako Pure Chemical Industries Ltd., Osaka, Japan) and EF-2001 (250 mg/kg) were dissolved in drinking water. Mice were given drinking water containing DSS ad libitum for seven days to induce colitis. Diazepam (DZP; 1 mg/kg; Wako Pure Chemical Industries Ltd.) and tandospirone (TDS; 1 or 3 mg/kg; Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) were dissolved in 0.5% Tween-20 (Vehicle; Wako Pure Chemical Industries Ltd.). EF-2001 was administered orally (per os [p.o.]) from 14 days before the beginning of DSS administration until the day prior to the last DSS treatment. DZP and TDS were administered intraperitoneally (i.p.) 30 min before the behavioral tests on day seven after the start of DSS administration. The dose for each drug used was calculated from previous reports32,41,42,43,44.
Evaluation of colon inflammation
This evaluation was conducted according to the experimental protocol shown in Figs. 1a and 5a. Disease Activity Index (DAI) scores correlate well with pathological findings in the DSS-induced IBD model45. DAI scores were obtained based on methods described in prior publications30,31,32,46. DAI is a score for each of stool consistency and bleeding, as detailed in Supplemental Table S1. The length of the colon, from above the anus to the top of the cecum, was measured after the mice had been euthanized.
Effect of DSS concentration on ulcerative colitis-like findings and anxiety-like behaviors. (a) Experimental time course for assessment of inflammation, behavioral tests, western blotting, ELISA, and immunohistochemical of experimental protocols. Changes in rectal bleeding (b), stool consistency (c), colon length (d), head-dip counts (e), head-dip duration (f), total time spent in the central area (g), number of open-arm entries (h), percentage of distance traveled (i) or time spent (j) in the open arm in dextran sulfate sodium (DSS)-treated mice on day seven. Bars represent means ± standard error of mean (SEM). *p < 0.05 and **p < 0.01 vs. water group (n = 14 per group).
Hole-board test
The hole-board test was carried out according to the method reported in prior research47,48,49. To investigate the changes in anxiety-like behaviors, the exploratory behaviors of mice on the hole-board, that is, the number or duration of head-dips, total time spent in the central area, and moving distance, were tested using an automatic hole-board apparatus (model ST-1; Muromachi Kikai, Tokyo, Japan). The apparatus consisted of a gray wooden box (50 cm × 50 cm × 50 cm) with four equidistant holes 3 cm in diameter in the floor50. Each animal was placed in the center of the hole-board and allowed to freely explore the apparatus over a 5-min period. Experiments were recorded with a video camera through a custom designed interface (DV-Trach; Muromachi Kikai). All of the data were analyzed and stored in a personal computer using analytical software (Comp ACT HBS; Muromachi Kikai).
Elevated plus-maze test
To investigate changes in anxiety-like behavior, mice were tested using the elevated plus-maze paradigm (EPM-04M; Muromachi Kikai). The apparatus was elevated 40 cm from the ground and the maze consisted of two opposing open arms (30 cm × 6 cm × 0.3 cm) and two opposing enclosed arms (30 cm × 6 cm × 15 cm) that were connected by a central platform (6 cm × 6 cm, 70 lx), thus forming the shape of a plus sign. Each mouse was placed on a left front corner, and the distance that the mouse moved in the apparatus was recorded for 5 min by an overhead color CCD camera that tracked the center of the mouse. Moreover, the number of entries into and the time spent in open or enclosed arms were also recorded. Data from the CCD camera were collected through a custom designed interface (CAT-10; Muromachi Kikai) as a reflection signal. All of the data were analyzed and stored in a personal computer using analytical software (Comp ACT HBS; Muromachi Kikai). The results were calculated as mean ratios of the time spent or distance in the open arms to the total time spent or distance in both the open and enclosed arms. Moreover, the total number of entries into the open arms was also measured.
Western blotting
Western blotting was performed according to the experimental protocol shown in Figs. 1a and 5a. Brain samples were collected from mice on day seven of DSS treatment. Moreover, mice were treated with water or EF-2001 for 20 days and sacrificed by decapitation 24 h after the last administration. The brain was immediately removed and the PFC, hypothalamus, amygdala, or ventral hippocampus were dissected quickly by a mouse brain slicer (Muromachi Kikai, Tokyo, Japan) to produce coronal sections of 1 mm thickness. We used samples from mice that had not undergone any behavioral tests. The brain atlas of Paxinos and Franklin was used as a reference to guide all dissections51. Protein isolation and western blotting were performed as described in prior works52,53,54. After electrophoresis, proteins were transferred electrically from the gel onto a polyvinylidene difluoride membrane using a semi-dry blotting apparatus (Bio-Rad Laboratories, Hercules, CA, USA). The blots were blocked for 30 min with 5% skim-milk in Tris-buffered saline supplemented with 0.01% Tween-20 (TBST). Subsequently, membranes were cut between 50 and 75 kDa, and probed with antibodies except for p-CAMKII, t-CAMKII, and BDNF against phosphorylated (p)-GR (Ser211) (1:1000; Cell Signaling Technology, Danvers, MA, USA, #4161), total (t)-GR (1:200; Santa Cruz Biotechnology, Dallas, TX, USA, sc-393232), NR1 (1:200; Cell Signaling Technology, #4204), NR2A (1:200; Cell Signaling Technology, #4205), NR2B (1:500; Cell Signaling Technology, #4207), p-CAMKII (1:1000; Cell Signaling Technology, #3361), t-CAMKII (1:1000; Cell Signaling Technology, #3362), p-CREB (1:500; Cell Signaling Technology, #9198), t-CREB (1:1000; Cell Signaling Technology, #9197), BDNF (1:1000; Abcam Ltd., Cambridge, UK, ab108319), synaptophysin (1:2000; Sigma-Aldrich, St. Louis, USA, S5768), synaptosomal-associated protein 25 (SNAP25; 1:1000; Santa Cruz Biotechnology, sc-7539), postsynaptic density protein 95 (PSD95; 1:1000; Cell Signaling Technology, #3450), drebrin (1:200; Medical & Biological Laboratories Co., Ltd., Tokyo, Japan, D029-3), microtubule-associated protein 2 (MAP2; 1:2000; Sigma-Aldrich, M9942), neuronal nuclei (NeuN; 1:500; Millipore, MAB377), and β-actin (1:1000; Santa Cruz Biotechnology, sc-47778) overnight at 4 °C. The blots were washed several times and then incubated at room temperature for 1 h with a secondary antibody (horseradish peroxidase-conjugated anti-rabbit, anti-goat, or anti-mouse immunoglobulin G (IgG) antibody diluted 1:10,000 with TBST containing 5% skim-milk). Blots were developed using an enhanced chemiluminescence assay kit (GE Healthcare, Buckinghamshire, UK) or ImmunoCruz (Santa Cruz Biotechnology) and scanned, optimized, and analyzed using the Quantity One 1-D Analysis Software version 4.5.2 (Bio-Rad Laboratories). The density of the corresponding bands was analyzed using Image Studio Lite version 5.2 (LI-COR Biosciences, Lincoln, NE, USA).
Measurement of serum LPS and corticosterone concentration
ELISA was performed according to the experimental protocol shown in Fig. 5a. Mice were treated with water or EF-2001 for 20 days and decapitated 24 h after the last treatment. The blood samples were collected from the decapitated mice into spitz tubes for serum isolation (Eiken Chemical Co., Ltd., Tokyo, Japan, #HC1100), centrifuged at 4 °C, 15 min, 2380 × g, and the supernatant was collected as a serum sample and stored at − 80 °C until the day of measurement. LPS and corticosterone concentrations were quantified using the Mouse Anti-LPS IgG Antibody Assay Kit (Chondrex, USA, WA, #6106) and Corticosterone ELISA Kit AssayMax (AssayPro, USA, MO, #EC3001-1), respectively.
Immunohistochemical study
Immunohistochemical study was performed according to the experimental protocol shown in Fig. 5a. Mice were sacrificed 24 h after the last administration of EF-2001. Brain samples were collected as described in prior works41,42,55,56. The brains were cut into 50-μm sections from the bregma − 1.40 mm to − 2.00 mm using a cryostat (Leica CM3050, Leica Biosystems, Tokyo, Japan).
Frozen sections were mounted on glass slides (Matsunami Glass, Osaka, Japan). After washing three times every 5 min, the sections were incubated with PBS containing 1% normal goat serum (Life Technologies Corporation, Carlsbad, CA, USA) or 3% bovine serum albumin and 0.3% Triton X-100 (PBSGT or PBSBT) at room temperature (23 ± 1 °C) for 2 h. The sections were incubated overnight at 4 °C with rabbit anti-p-GR (Ser211) (1:200; Cell Signaling Technology, #4161), rabbit anti-p-CAMKII (1:500; Cell Signaling Technology, #3361), rabbit anti-p-CREB (1:200; Cell Signaling Technology, #9198), mouse anti-NeuN (1:200; Millipore, MAB377), mouse anti-glial fibrillary acidic protein (GFAP; 1:200; Millipore, MAB360), or goat anti-ionized calcium-binding adaptor molecule 1 (Iba1; 1:200, Abcam, ab5076). Sections were washed and incubated overnight at 4 °C with goat anti-rabbit IgG Alexa Fluor 488 (1:200; Molecular Probes, Eugene, OR, USA, A-11008), goat anti-mouse IgG Alexa Fluor 568 (1:200; Molecular Probes, A-11004), Cy3-conjugated donkey anti-goat IgG (1:200; Jackson ImmunoResearch Inc., PA, West Grove, USA, 705-165-147), or FITC-conjugated donkey anti-rabbit IgG (1:200; Jackson ImmunoResearch, 711-095-152) with PBSGT or PBSBT, and with 4′,6-diamidino-2-phenylindole (DAPI, 1:100; Wako Pure Chemical Industries, Ltd) to identify the nuclei. Finally, sections were washed and covered with a cover slip with VECTASHIELD Mounting Medium (Vector Laboratories, Newark, CA, USA). Labeled sections were analyzed using a confocal laser-scanning microscope (FV1200; OLYMPUS, Tokyo, Japan).
Statistical analysis
The results of the experiments are expressed as mean ± standard error of the mean (SEM). The statistical significance of differences was determined using Student's t-test for two-group comparisons. The significance of differences was determined via one- or two-way analysis of variance (ANOVA), followed by the Tukey–Kramer test or Bonferroni test for multiple-group comparisons. The statistical significance of differences in DAI scores was assessed using a non-parametric Mann–Whitney test for two-group comparisons or a non-parametric Kruskal–Wallis test followed by the Dunn test for multiple-group comparisons. The criterion of significance was set at p < 0.05. When the main effect of group or time was significant without interaction, we performed an exploratory and limited pairwise post-hoc comparison, consistent with our a priori hypothesis. All statistical analyses using GraphPad Prism 7 (GraphPad Software, San Diego, CA, USA) were performed by investigators other than the experimenters to avoid bias and ensure blinding.
Ethics approval and consent to participate
All experiments were approved by the Ethics Committee of Animal Experiments at the International University of Health and Welfare (Ohtawara, Japan; approval number: 19015, 22009). All animal experiments complied with the ARRIVE guidelines and were performed in accordance with the guidelines established by the Animal Research Committee of the International University of Health and Welfare and the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals. Efforts were made to minimize the suffering of the animals and reduce the number of animals used in the experiments.
Results
Concentration-dependent effect of DSS on DAI scores, colon length, and anxiety-related behaviors in mice
The DAI scores for both stool consistency and rectal bleeding in DSS-treated mice (1.5%, 2%, or 2.5%) were significantly higher compared with those in the control group [Kruskal–Wallis test: p < 0.0001, Fig. 1b, p < 0.0001, Fig. 1c]. The colon length in DSS-treated mice (1.5%, 2%, or 2.5%) was significantly shorter compared to that in control group mice [one-way ANOVA: F (3, 52) = 300.6, p < 0.0001, Fig. 1d]. In the hole-board test, compared to the control group, the 2% DSS-treated group showed a significant decrease in the number of head-dips and the time spent in the central area (Fig. 1e,g), and the 2.5% DSS-treated group showed a significant decrease in performance across all items: the number or duration of head-dips and total time spent in the central area [one-way ANOVA: F (3, 52) = 5.034, p = 0.0039, Fig. 1e; F (3, 52) = 3.321, p = 0.0267, Fig. 1f; F (3, 52) = 14.56, p < 0.0001, Fig. 1g]. Furthermore, in the elevated plus-maze test, there were significant reductions in the number of open-arm entries as well as the percentage of distance traveled or time spent in the open arm in the 2% and 2.5% DSS treatment groups, compared to the control group [one-way ANOVA: F (3, 52) = 24.38, p < 0.0001, Fig. 1h; F (3, 52) = 21.71, p < 0.0001, Fig. 1i; F (3, 52) = 7.943, p = 0.0002, Fig. 1j].
Mice treated with 2.5% DSS were observed to move less, based on the distance covered, than controls (Supplementary Fig. S1). To exclude the possibility of decreased locomotor activity associated with exacerbated IBD-like symptoms, the concentration of 2% DSS was used in subsequent experiments in this study of anxiety associated with IBD.
Time-dependent effects of DSS on DAI scores, colon length, and anxiety-related behaviors in mice
As shown in Fig. 2, diarrhea, bloody stool, and shortened colon length were observed on days five and seven of 2% DSS treatment, but not on day three [two-way ANOVA: time: F (2, 114) = 38.55, p < 0.0001, group: F (1, 114) = 137.7, p < 0.0001, time × group: F (2, 114) = 38.55, p < 0.0001, Fig. 2a; time: F (2, 114) = 30.65, p < 0.0001, group: F (1, 114) = 95.6, p < 0.0001, time × group: F (2, 114) = 34.12, p < 0.0001, Fig. 2b; time: F (2, 114) = 23.14, p < 0.0001, group: F (1, 114) = 177.2, p < 0.0001, time × group: F (2, 114) = 30.68, p < 0.0001, Fig. 2c]. In contrast, reductions in the number or duration of head-dips, the time spent in the central area, the number of open-arm entries, and the percentage of distance traveled or time spent in the open arm were observed on day seven of 2% DSS treatment, but not on days three and five [two-way ANOVA: time: F (2, 114) = 0.914, p = 0.4038, group: F (1, 114) = 10.13, p = 0.0019, time × group: F (2, 114) = 3.74, p = 0.0267, Fig. 2d; time: F (2, 114) = 0.8254, p = 0.4407, group: F (1, 114) = 4.778, p = 0.0309, time × group: F (2, 114) = 1.083, p = 0.3420, Fig. 2e; time: F (2, 114) = 5.197, p = 0.0069, group: F (1, 114) = 17.91, p < 0.0001, time × group: F (2, 114) = 5.674, p = 0.0045, Fig. 2f; time: F (2, 114) = 3.989, p = 0.0212, group: F (1, 114) = 4.793, p = 0.0306, time × group: F (2, 114) = 3.561, p = 0.0316, Fig. 2g; time: F (2, 114) = 4.482, p = 0.0134, group: F (1, 114) = 4.952, p = 0.0280, time × group: F (2, 114) = 4.402, p = 0.0144, Fig. 2h; time: F (2, 114) = 1.196, p = 0.3062, group: F (1, 114) = 15.85, p = 0.0001, time × group: F (2, 114) = 3.353, p = 0.0384, Fig. 2i]. Based on these results, day seven after the commencement of 2% DSS treatment was found to be the best time point to investigate changes in the IBD model with anxiety.
Time-course of DSS treatment for ulcerative colitis-like findings and anxiety-like behaviors. Time-course of rectal bleeding (a), stool consistency (b), colon length (c), head-dip counts (d), head-dip duration (e), total time spent in the central area (f), number of open-arm entries (g), percentage of distance traveled (h) or time spent (i) in the open arm in dextran sulfate sodium (DSS; 1.5%)-treated mice on days three, five, and seven. Bars represent means ± standard error of mean (SEM). *p < 0.05 and **p < 0.01 vs. water group (n = 20 per group).
Effects of DZP or TDS on anxiety-related behaviors in 2% DSS-treated mice
The effects of DZP and TDS—clinically used anxiolytic drugs—on anxiety-like behaviors in DSS-treated mice were examined. The results showed that acute administration of DZP (1 mg/kg) led to significant improvements in the outcomes assessed, effectively addressing reductions in the number or duration of head-dips, the time spent in the central area, the number of open-arm entries, and the percentage of distance traveled or time spent in the open arm in the 2% DSS-treated group in the hole-board and elevated plus-maze tests [one-way ANOVA: F (4, 67) = 17.52, p < 0.0001, Fig. 3a; F (4, 67) = 10.33, p < 0.0001, Fig. 3b; F (4, 67) = 5.942, p = 0.0004, Fig. 3c; F (4, 67) = 16.63, p < 0.0001, Fig. 3e; F (4, 67) = 14.12, p < 0.0001, Fig. 3f; F (4, 67) = 7.654, p < 0.0001, Fig. 3g]. In contrast, acute administration of TDS (1, 3 mg/kg) had a significant restorative effect only with regard to the decreased time spent in the central area, with no effect on other changes in the hole-board and elevated plus-maze tests (Fig. 3c,d).
Effects of anxiolytics on DSS-induced anxiety-like behaviors. Effect of acute treatment with diazepam (DZP) or tandospirone (TDS) on head-dip counts (a), head-dip duration (b), total time spent in the central area (c), number of open-arm entries (e), percentage of distance traveled (f) or time spent (g) in the open arm in dextran sulfate sodium (DSS)-treated mice. (d) and (h) Representative activity traces in the hole-board test (d) and the elevated plus-maze test (h). The central area in the hole-board test is indicated by a green rectangle. Bars represent means ± standard error of mean (SEM). **p < 0.01 vs. water-treated water group, #p < 0.05 and ##p < 0.01 vs. water-treated DSS group (n = 13–16 per group).
Changes in phosphorylation levels of GR in the brain of 2% DSS-treated mice
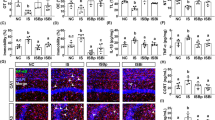
We examined the changes in phosphorylation levels of GR in the brain regions that are closely related to the development of anxiety, such as the PFC, amygdala, hypothalamus, and ventral hippocampus. According to the results, 2% DSS-treated mice showed an increase in phosphorylation levels of GR in the PFC and ventral hippocampus, but not in the amygdala and hypothalamus, compared to the water group (Student’s t-test: t = 3.125, df = 10, p = 0.0108, Fig. 4a; t = 1.709, df = 10, p = 0.1182, Fig. 4b; t = 1.05, df = 10, p = 0.3182, Fig. 4c; t = 1.255, df = 10, p = 0.2381, Fig. 4d; t = 1.379, df = 10, p = 0.1980, Fig. 4e; t = 0.8407, df = 10, p = 0.4201, Fig. 4f; t = 4.181, df = 10, p = 0.0019, Fig. 4g; t = 2.02, df = 10, p = 0.0710, Fig. 4h).
Changes in phosphorylation levels of glucocorticoid receptors in anxiety-related brain regions. Changes in phosphorylation and expression levels of glucocorticoid receptor (GR) in the prefrontal cortex (PFC) (a,b), amygdala (c,d), hypothalamus (e,f), and ventral hippocampus (g,h) of DSS-treated mice. Quantification of the normalized values of p-GR and t-GR with t-GR and β-actin, respectively. Original immunoblot images were shown in Supplementary Fig. S2. Bars represent means ± standard error of mean (SEM). *p < 0.05 and **p < 0.01 vs. water group (n = 6 per group).
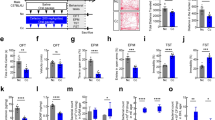
Effects of EF-2001 on DAI scores, colon length, and anxiety-related behaviors in mice
EF-2001 prevented DSS-induced diarrhea, bloody stool, and colon atrophy in mice [Kruskal–Wallis test: p < 0.0001, Fig. 5b, p < 0.0001, Fig. 5c. One-way ANOVA: F (2, 42) = 99.96, p < 0.0001, Fig. 5d]. Moreover, administration of EF-2001 in DSS-treated mice prevented reductions in the number or duration of head-dips and the time spent in the central area in the hole-board test [one-way ANOVA: F (2, 42) = 14.98, p < 0.0001, Fig. 5f; F (2, 42) = 10.82, p = 0.0002, Fig. 5g; F (2, 42) = 8.529, p = 0.0008, Fig. 5h]. In contrast, DSS-induced reductions in the number of open-arm entries and the percentage of distance traveled or time spent in the open arm in the elevated plus-maze test were not prevented by the administration of EF-2001 [one-way ANOVA: F (2, 42) = 44.88, p < 0.0001, Fig. 5j; F (2, 42) = 36.04, p < 0.0001, Fig. 5k; F (2, 42) = 37.68, p < 0.0001, Fig. 5l].
Effects of EF-2001 on DSS-induced ulcerative colitis-like findings and anxiety-like behaviors. (a) Experimental time course for assessment of inflammation, behavioral tests, western blotting, ELISA, and immunohistochemical of experimental protocols. Effects of chronic treatment with Enterococcus faecalis 2001 (EF-2001) on rectal bleeding (b), stool consistency (c), colon length (d), head-dip counts (f), head-dip duration (g), total time spent in the central area (h), the number of open-arm entries (j), percentage of distance traveled (k) or time spent (l) in the open arm in dextran sulfate sodium (DSS)-treated mice. (e) Representative colon images from each group are shown. (i) and (m) Representative activity traces in the hole-board test (i) and the elevated plus-maze test (m). The central area in the hole-board test is indicated by a green rectangle. Bars represent means ± standard error of mean (SEM). **p < 0.01 vs. water-treated water group, #p < 0.05 and ##p < 0.01 vs. water-treated DSS group (n = 15 per group).
Effects of EF-2001 on serum LPS and corticosterone concentration and p-GR in the brain
Serum corticosterone levels in the 2% DSS-treated mice were significantly elevated compared to those in the water group (serum LPS concentration in the 2% DSS-treated group was slightly higher (p = 0.1096) compared to that in the water group), while these changes were prevented by EF-2001 administration [one-way ANOVA: F (2, 21) = 3.775, p = 0.0398, Fig. 6a; F (2, 21) = 9.151, p = 0.0014, Fig. 6b]. Moreover, administration of EF-2001 suppressed the increase of p-GR (Ser211) levels in the PFC of 2% DSS-treated mice, but not in the ventral hippocampus [one-way ANOVA: F (2, 16) = 10.78, p = 0.0011, Fig. 6c; F (2, 16) = 4.528, p = 0.0277, Fig. 6d]. Additionally, we observed that the p-GR (Ser211) labeling was colocalized in the neurons, but not in the astrocytes and microglia, of the PFC of DSS-treated mice (Fig. 6e).
Effects of EF-2001 on serum LPS and corticosterone, and p-GR in the brain. Effects of chronic treatment with Enterococcus faecalis 2001 (EF-2001) on increased serum LPS (a) and corticosterone (b) concentration, and enhanced p-GR in the prefrontal cortex (PFC) (c) and ventral hippocampus (d) of DSS-treated mice. Quantification of the normalized values of p-GR with t-GR. Original immunoblot images were shown in Supplementary Fig. S3. (e) Microscopy images of p-GR (Ser211) (green), DAPI (blue), and NeuN, GFAP or Iba1 (red) immunostaining in the PFC of DSS-treated mice. Bars represent means ± standard error of mean (SEM). *p < 0.05 and **p < 0.01 vs. water-treated water group, #p < 0.05 and ##p < 0.01 vs. water-treated DSS group (n = 6–8 per group).
Effects of EF-2001 on NMDAR signaling and synaptic plasticity-related proteins
As shown in Fig. 7, the expression levels of NR2A and NR2B in the PFC of 2% DSS-treated mice were significantly lower compared to those in the water group (the expression levels of p-CAMKII and BDNF in the PFC of the 2% DSS-treated group decreased slightly (p-CAMKII: p = 0.0755; BDNF: p = 0.052) compared to those in the water group), whereas these changes were reversed by EF-2001 administration [one-way ANOVA: F (2, 15) = 15.01, p = 0.0003, Fig. 7b; F (2, 15) = 5.715, p = 0.0143, Fig. 7c; F (2, 15) = 8.886, p = 0.0028, Fig. 7d; F (2, 15) = 6.756, p = 0.0081, Fig. 7f]. Furthermore, EF-2001 treatment increased the expression levels of p-CREB and drebrin in the PFC of 2% DSS-treated mice [one-way ANOVA: F (2, 15) = 23.33, p < 0.0001, Fig. 7e; F (2, 15) = 19.03, p < 0.0001, Fig. 7j]. The expression levels of NR1, synaptophysin, SNAP25, PSD95 MAP2, and NeuN were not significantly different among the three groups [one-way ANOVA: F (2, 15) = 0.07509, p = 0.9280, Fig. 7a; F (2, 15) = 1.015, p = 0.3861, Fig. 7g; F (2, 15) = 0.8834, p = 0.4338, Fig. 7h; F (2, 15) = 0.4222, p = 0.6632, Fig. 7i; F (2, 15) = 0.6027, p = 0.5601, Fig. 7k; F (2, 15) = 1.181, p = 0.3339, Fig. 7l]. Additionally, we observed that the p-CAMKII and p-CREB labeling were colocalized in the neurons of the PFC (Fig. 7m,n).
Effects of EF-2001 on NMDAR signaling and synaptic plasticity-related proteins in the PFC. Effects of chronic treatment with Enterococcus faecalis 2001 (EF-2001) on the NMDA receptor subunits (NRs), CAMKII/CREB/BDNF pathway, and synaptic plasticity-related proteins. a-l: Quantification of the normalized values of NR1 (a), NR2A (b), NR2B (c), BDNF (f), synaptophysin (g), SNAP25 (h), PSD95 (i), drebrin (j), MAP2 (k), and NeuN (l) levels with β-actin and p-CAMKII (d) and p-CREB (e) with t-CAMKII and t-CREB, respectively. Original immunoblot images were shown in Supplementary Fig. S4. (m) and (n) Microscopy images of NeuN (red), DAPI (blue), and p-CAMKII (m) or p-CREB (n) (green) immunostaining in the PFC of DSS mice treated with EF-2001. Bars represent means ± standard error of mean (SEM). *p < 0.05 and **p < 0.01 vs. water-treated water group, #p < 0.05 and ##p < 0.01 vs. water-treated DSS group (n = 6 per group).
Discussion
A high prevalence of psychiatric disorders such as depression and anxiety has been reported among patients with IBD, but the mechanisms underlying the relationship between intestinal inflammation and anxiety symptoms remain unclear. In this study, we examined the effects of EF-2001 on IBD-like physiological changes and anxiety-like behaviors in DSS-treated mice. The results revealed that chronic administration of EF-2001 suppressed these changes. Furthermore, EF-2001 administration in DSS-treated mice suppressed the increase in serum LPS and corticosterone, the increase in GR phosphorylation, and the decrease in NR2A and NR2B expression in the PFC, and promoted the CAMKII/CREB/BDNF-Drebrin pathways in the PFC of DSS-treated mice. To the best of our knowledge, this is the first study to show that the anxiolytic effect of EF-2001 may be involved in the suppression of GR activity in the PFC via reduction of colon-derived LPS release and in synaptic plasticity via promotion of the CAMKII/CREB/BDNF-Drebrin pathways.
As mentioned above, a high rate of neuropsychiatric disorders has been reported to occur in patients with IBD1,2,3,4,5, but the underlying mechanisms remain unclear; other studies have reported that DSS-treated mice, commonly used in modeling UC, show UC findings, such as diarrhea and bloody stools, and exhibit anxiety-like behaviors28,29. In the present study, 2% DSS-treated mice exhibited UC-like symptoms such as diarrhea, hematochezia, and shortened colon length as well as anxiety-like behaviors in the hole-board and elevated plus-maze tests (Figs. 1, 2), without affecting locomotor activity (Supplementary Fig. S1). The effects of the clinically used anxiolytics DZP and TDS on anxiety-like behaviors in DSS-treated mice were also examined. The results showed that acute administration of DZP suppressed anxiety-like behaviors observed in the 2% DSS-treated group in both the hole-board and elevated plus-maze tests, while acute administration of TDS suppressed only the decrease in behavioral time in the central area in the hole-board test in the 2% DSS-treated group, but had no effect on other anxiety-like behaviors (Fig. 3). In clinical practice, TDS is less effective as a single dose than DZP and more effective when administered chronically; the results of this study appear to reflect this clinical fact57. Activation of GR in the PFC, hypothalamus, amygdala, and ventral hippocampus is known to induce anxiety-like behaviors via diverse mechanisms12,13,14,15. DSS-treated mice showed increased phosphorylation levels of GR in the PFC and ventral hippocampus (Fig. 4). From these results, we suggest that DSS-treated mice are a useful model of anxiety accompanying UC, as demonstrated the three validities (surface, predictive, and construct validities) which must be fulfilled when building a model animal58,59. Moreover, it has been suggested that increased phosphorylation of GR in the PFC and ventral hippocampus may be implicated in anxiety-like behaviors.
Previous studies demonstrated that EF-2001 administration prevented DSS-induced depression-like behavior and UC-like symptoms, with inflammation suppressed in the hippocampus and rectum32. In the present study, EF-2001 prevented DSS-induced UC-like symptoms and anxiety-like behaviors in the hole-board test, while it did not suppress anxiety-like behaviors in the elevated plus-maze test (Fig. 5). Moreover, administration of EF-2001 showed no changes in normal mice in both the hole-board and elevated plus-maze tests (Supplementary Fig. S5). As the elevated plus-maze test is thought to assess anxiety-like behavior under strong fear conditions compared to the hole-board test60,61, it may be that EF-2001 administration does not mitigate anxiety-like behavior associated with fear, but only that caused by mild stress.
In patients with UC, the intestinal microbiota is thought to be imbalanced, with a relative increase in gram-negative bacteria and a decrease in tight junctions between cells in the intestinal tract, leading to increased migration of inflammatory cytokines and LPS secreted by gram-negative bacteria into the blood7,8. In animal studies, intraperitoneal administration of LPS has been reported to increase blood corticosterone9,10,11. In the present study, DSS-treated mice were found to have increased serum LPS levels and a significant increase in serum corticosterone levels, and these changes were significantly prevented by EF-2001 administration (Fig. 6a,b). These results suggest that UC-like symptoms caused LPS to migrate from the intestinal tract into the blood and elevated the blood corticosterone levels. Moreover, EF-2001 administration prevented an increase in p-GR levels in the PFC of DSS-treated mice, but not in the ventral hippocampus (Fig. 6c,d). We found that phosphorylation of GR was localized to neurons in the PFC, but not to astrocytes and microglia (Fig. 6e). The ventral hippocampus region is known to be related to anxiety under conditions involving fearful stimuli, which can lead to high stress62,63. In the present study, EF-2001 showed no anxiolytic effect in the elevated plus-maze test, which assesses anxiety under fearful conditions. This result may be related to the fact that EF-2001 did not suppress the increase in phosphorylation levels of GR in the ventral hippocampus. From these findings, we suggest that the anxiolytic effect of EF-2001 may be associated with the decreased phosphorylation level of GR in the PFC neurons via the prevention of the increase in serum LPS and corticosterone levels.
Activation of GR by long-term stress exposure has been reported to cause abnormal synaptic plasticity16. Increased corticosterone causes decreased expression levels of NR2A, NR2B, BDNF, and synaptic plasticity-related proteins17,18,19, and these changes in the PFC are associated with the development of anxiety-like behavior20,21,22. Activated NMDAR phosphorylates CAMKII, which in turn phosphorylates CREB, resulting in BDNF and drebrin translation through CREB activation23,24,25. Activation of BDNF signaling by the tropomyosin receptor kinase B agonist 7,8-dihydroxyflavone produces anxiolytic effects26. It has been reported that increased expression of drebrin in the PFC plays an important role in anxiolytic effects27. BDNF and Drebrin expression in the PFC has been suggested to correlate with anxiety-like behaviors in rodents27,64,65. In the present study, the expression levels of p-CAMKII and BDNF in the PFC of DSS-treated mice showed a decreasing tendency, and the expression levels of NR2A and NR2B significantly decreased, while these changes were reversed by EF-2001 treatment (Fig. 7b–d,f). Furthermore, EF-2001 treatment increased the expression levels of p-CREB and drebrin in the PFC of DSS-treated mice (Fig. 7e,j). In contrast, the expression levels of NR1, synaptophysin, SNAP25, PSD95, MAP2, and NeuN in the PFC were not significantly altered. We found that p-CAMKII and p-CREB were localized to neurons in the PFC (Fig. 7m,n). From these findings, we suggest that the anxiolytic effects of EF-2001 may be associated with activation of the CAMKII/CREB/BDNF-Drebrin pathways in the PFC neurons via normalized expression levels of NR2A and NR2B.
Despite these novel results, this study had some limitations that must be noted. In the present study, we focused on the relationship between anxiety behavior and activation of GR in the brain. However, we consider that studies focusing on these relationships alone are insufficient to elucidate the pathogenesis of anxiety accompanying UC. The reason is that there have been many studies focusing on neural activity in the brain and anxiety behavior66,67,68,69, which we did not examine in this study. Moreover, it is unclear as to how elevated serum LPS contributes to elevated corticosterone and how EF-2001 acts on changes in serum LPS. Furthermore, it has been reported that changes in the intestinal microbiota affect the brain via the vagus nerve, resulting in anxiety-like behavior70. To determine whether the microbiota-gut-brain axis via the vagus nerve contributes to the anxiolytic effects of EF-2001, it is necessary to examine changes in the gut microbiota and the effects of vagotomy. These issues will be clarified in future studies.
Conclusion
As summarized in Fig. 8, the results from our DSS-induced UC model with mice show that elevated serum LPS and corticosterone levels activate GR in the PFC, resulting in anxiety-like behaviors. EF-2001 inhibits these changes by preventing enteritis symptoms, and produces anxiolytic effects via the activation of synaptic plasticity in the PFC. These results suggest a close relationship between IBD and anxiety, and provide important evidence for a mechanism by which the attenuation of intestinal inflammatory symptoms in UC may reduce the risk of psychiatric disorders.
Data availability
The datasets used and/or analyzed in the current study are available from the corresponding author on reasonable request.
Abbreviations
- ANOVA:
-
Analysis of variance
- BDNF:
-
Brain-derived neurotrophic factor
- BRM:
-
Biological response modifier
- CAMKII:
-
Calcium/calmodulin-dependent protein kinase II
- CREB:
-
CAMP response element binding protein
- DAI:
-
Disease activity index
- DZP:
-
Diazepam
- DSS:
-
Dextran sulfate sodium
- EF-2001:
-
Enterococcus Faecalis 2001
- ELISA:
-
Enzyme-linked immunosorbent assay
- GFAP:
-
Glial fibrillary acidic protein
- GR:
-
Glucocorticoid receptor
- Iba1:
-
Ionized calcium-binding adapter molecule 1
- IBD:
-
Inflammatory bowel disease
- IgG:
-
Immunoglobulin G
- i.p.:
-
Intraperitoneally
- LPS:
-
Lipopolysaccharides
- MAP2:
-
Microtubule-associated protein 2
- NeuN:
-
Neuronal nuclei
- NMDAR:
-
N-Methyl-d-aspartate receptor
- NR:
-
N-Methyl-d-aspartate receptor subunit
- PBS:
-
Phosphate-buffered saline
- PBSBT:
-
PBS containing 3% bovine serum albumin and 0.3% Triton X-100
- PBSGT:
-
PBS containing 1% normal goat serum and 0.3% Triton X-100
- p.o.:
-
Per os
- PFC:
-
Prefrontal cortex
- PSD95:
-
Postsynaptic density protein 95
- SEM:
-
Standard error of the mean
- SNAP25:
-
Synaptosomal-associated protein 25
- TBST:
-
Tris-buffered saline supplemented with 0.01% Tween-20
- TDS:
-
Tandospirone
- UC:
-
Ulcerative colitis
References
Graff, L. A., Walker, J. R. & Bernstein, C. N. Depression and anxiety in inflammatory bowel disease: A review of comorbidity and management. Inflamm. Bowel Dis. 15, 1105–1118. https://doi.org/10.1002/ibd.20873 (2009).
Kurina, L. M., Goldacre, M. J., Yeates, D. & Gill, L. E. Depression and anxiety in people with inflammatory bowel disease. J. Epidemiol. Community Health 55, 716–720. https://doi.org/10.1136/jech.55.10.716 (2001).
Lydiard, R. B. Irritable bowel syndrome, anxiety, and depression: What are the links?. J. Clin. Psychiatry 62(Suppl 8), 38–45 (2001) (discussion 46–37).
Mikocka-Walus, A. A. et al. Antidepressants and inflammatory bowel disease: A systematic review. Clin. Pract. Epidemiol. Ment. Health 2, 24. https://doi.org/10.1186/1745-0179-2-24 (2006).
Perez-Pardo, P., Hartog, M., Garssen, J. & Kraneveld, A. D. Microbes tickling your tummy: The importance of the gut–brain axis in Parkinson’s disease. Curr. Behav. Neurosci. Rep. 4, 361–368. https://doi.org/10.1007/s40473-017-0129-2 (2017).
Quaglio, A. E. V., Grillo, T. G., De Oliveira, E. C. S., Di Stasi, L. C. & Sassaki, L. Y. Gut microbiota, inflammatory bowel disease and colorectal cancer. World J. Gastroenterol. 28, 4053–4060. https://doi.org/10.3748/wjg.v28.i30.4053 (2022).
Capuco, A. et al. Current perspectives on gut microbiome dysbiosis and depression. Adv. Ther. 37, 1328–1346. https://doi.org/10.1007/s12325-020-01272-7 (2020).
Li, W. et al. TRPV4 inhibitor HC067047 produces antidepressant-like effect in LPS-induced depression mouse model. Neuropharmacology 201, 108834. https://doi.org/10.1016/j.neuropharm.2021.108834 (2021).
Mouihate, A. et al. Early life activation of toll-like receptor 4 reprograms neural anti-inflammatory pathways. J. Neurosci. 30, 7975–7983. https://doi.org/10.1523/JNEUROSCI.6078-09.2010 (2010).
Sylvia, K. E. & Demas, G. E. Acute intraperitoneal lipopolysaccharide influences the immune system in the absence of gut dysbiosis. Physiol. Rep. https://doi.org/10.14814/phy2.13639 (2018).
Girard-Joyal, O. et al. Age and sex differences in c-Fos expression and serum corticosterone concentration following LPS treatment. Neuroscience 305, 293–301. https://doi.org/10.1016/j.neuroscience.2015.06.035 (2015).
Scholl, J. L., Solanki, R. R., Watt, M. J., Renner, K. J. & Forster, G. L. Chronic administration of glucocorticoid receptor ligands increases anxiety-like behavior and selectively increase serotonin transporters in the ventral hippocampus. Brain Res. 1800, 148189. https://doi.org/10.1016/j.brainres.2022.148189 (2023).
Wei, Q. et al. Glucocorticoid receptor overexpression in forebrain: A mouse model of increased emotional lability. Proc. Natl. Acad. Sci. U. S. A. 101, 11851–11856. https://doi.org/10.1073/pnas.0402208101 (2004).
Piechota, M. et al. Glucocorticoid-regulated kinase CAMKIγ in the central amygdala controls anxiety-like behavior in mice. Int. J. Mol. Sci. https://doi.org/10.3390/ijms232012328 (2022).
Wang, D. C., Chen, T. J., Lin, M. L., Jhong, Y. C. & Chen, S. C. Exercise prevents the increased anxiety-like behavior in lactational di-(2-ethylhexyl) phthalate-exposed female rats in late adolescence by improving the regulation of hypothalamus–pituitary–adrenal axis. Horm. Behav. 66, 674–684. https://doi.org/10.1016/j.yhbeh.2014.09.010 (2014).
Picard, K. et al. Microglial-glucocorticoid receptor depletion alters the response of hippocampal microglia and neurons in a chronic unpredictable mild stress paradigm in female mice. Brain Behav. Immun. 97, 423–439. https://doi.org/10.1016/j.bbi.2021.07.022 (2021).
Dominguez, G. et al. Sustained corticosterone rise in the prefrontal cortex is a key factor for chronic stress-induced working memory deficits in mice. Neurobiol. Stress 10, 100161. https://doi.org/10.1016/j.ynstr.2019.100161 (2019).
Gourley, S. L., Kedves, A. T., Olausson, P. & Taylor, J. R. A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology 34, 707–716. https://doi.org/10.1038/npp.2008.123 (2009).
Liu, Y. et al. Corticosterone induced the increase of proBDNF in primary hippocampal neurons via endoplasmic reticulum stress. Neurotox. Res. 38, 370–384. https://doi.org/10.1007/s12640-020-00201-4 (2020).
Ren, W. et al. Embryonic ketamine produces a downregulation of prefrontal cortex NMDA receptors and anxiety-like behavior in adult offspring. Neuroscience 415, 18–30. https://doi.org/10.1016/j.neuroscience.2019.07.018 (2019).
Uysal, N. et al. Maternal exercise decreases maternal deprivation induced anxiety of pups and correlates to increased prefrontal cortex BDNF and VEGF. Neurosci. Lett. 505, 273–278. https://doi.org/10.1016/j.neulet.2011.10.039 (2011).
Li, Y. et al. (2R,6R)-hydroxynorketamine acts through GluA1-induced synaptic plasticity to alleviate PTSD-like effects in rat models. Neurobiol. Stress. 21, 100503. https://doi.org/10.1016/j.ynstr.2022.100503 (2022).
Moriguchi, S. et al. Reduced CaM kinase II and CaM kinase IV activities underlie cognitive deficits in NCKX2 heterozygous mice. Mol. Neurobiol. 55, 3889–3900. https://doi.org/10.1007/s12035-017-0596-1 (2018).
Yabuki, Y., Wu, L. & Fukunaga, K. Cognitive enhancer ST101 improves schizophrenia-like behaviors in neonatal ventral hippocampus-lesioned rats in association with improved CaMKII/PKC pathway. J. Pharmacol. Sci. 140, 263–272. https://doi.org/10.1016/j.jphs.2019.07.015 (2019).
Guo, G. et al. Testosterone modulates structural synaptic plasticity of primary cultured hippocampal neurons through ERK–CREB signalling pathways. Mol. Cell Endocrinol. 503, 110671. https://doi.org/10.1016/j.mce.2019.110671 (2020).
Baker-Andresen, D., Flavell, C. R., Li, X. & Bredy, T. W. Activation of BDNF signaling prevents the return of fear in female mice. Learn. Mem. 20, 237–240. https://doi.org/10.1101/lm.029520.112 (2013).
Temizer, R., Chen, Y. W. & Aoki, C. Individual differences in the positive outcome from adolescent ketamine treatment in a female mouse model of anorexia nervosa involve drebrin A at excitatory synapses of the medial prefrontal cortex. Synapse 77, e22253. https://doi.org/10.1002/syn.22253 (2023).
Cluny, N. L. et al. Recruitment of alpha4beta7 monocytes and neutrophils to the brain in experimental colitis is associated with elevated cytokines and anxiety-like behavior. J. Neuroinflamm. 19, 73. https://doi.org/10.1186/s12974-022-02431-z (2022).
Zhao, B. et al. Lycopene alleviates DSS-induced colitis and behavioral disorders via mediating microbes–gut–brain axis balance. J. Agric. Food Chem. 68, 3963–3975. https://doi.org/10.1021/acs.jafc.0c00196 (2020).
Nakagawasai, O. et al. Liver hydrolysate prevents depressive-like behavior in an animal model of colitis: Involvement of hippocampal neurogenesis via the AMPK/BDNF pathway. Behav. Brain Res. 390, 112640. https://doi.org/10.1016/j.bbr.2020.112640 (2020).
Takahashi, K. et al. Hippocampal and gut AMPK activation attenuates enterocolitis-like symptoms and co-occurring depressive-like behavior in ulcerative colitis model mice: Involvement of brain-gut autophagy. Exp. Neurol. 373, 114671. https://doi.org/10.1016/j.expneurol.2023.114671 (2023).
Takahashi, K. et al. Effect of Enterococcus faecalis 2001 on colitis and depressive-like behavior in dextran sulfate sodium-treated mice: involvement of the brain–gut axis. J. Neuroinflamm. 16, 201. https://doi.org/10.1186/s12974-019-1580-7 (2019).
Sudeep, H. V., Venkatakrishna, K., Raj, A., Reethi, B. & Shyamprasad, K. Viphyllin™, a standardized extract from black pepper seeds, mitigates intestinal inflammation, oxidative stress, and anxiety-like behavior in DSS-induced colitis mice. J. Food Biochem. 46, e14306. https://doi.org/10.1111/jfbc.14306 (2022).
Zhang, X. et al. Effects of alternate-day fasting, time-restricted fasting and intermittent energy restriction DSS-induced on colitis and behavioral disorders. Redox Biol. 32, 101535. https://doi.org/10.1016/j.redox.2020.101535 (2020).
Sagarwala, R., Malmstrom, T. & Nasrallah, H. A. Effects of nonpharmacological therapies on anxiety and cortisol: A meta-analysis. Ann. Clin. Psychiatry 30, 91–96 (2018).
Choi, E. J. et al. Effect of Enterococcus faecalis EF-2001 on experimentally induced atopic eczema in mice. Food Sci. Biotechnol. 25, 1087–1093. https://doi.org/10.1007/s10068-016-0175-7 (2016).
Choi, E. J. et al. Heat-killed Enterococcus faecalis EF-2001 ameliorates atopic dermatitis in a murine model. Nutrients 8, 146. https://doi.org/10.3390/nu8030146 (2016).
Gu, Y. H. et al. Pharmaceutical production of anti-tumor and immune-potentiating Enterococcus faecalis-2001 β-glucans: Enhanced activity of macrophage and lymphocytes in tumor-implanted mice. Curr. Pharm. Biotechnol. 18, 653–661. https://doi.org/10.2174/1389201018666171002130428 (2017).
Hamamoto, H., Ogasawara, A. A., Iwasa, M. & Sekimizu, K. Establishment of a polymerase chain reaction-based method for strain-level management of Enterococcus faecalis EF-2001 using species-specific sequences identified by whole genome sequences. Front. Microbiol. 13, 959063. https://doi.org/10.3389/fmicb.2022.959063 (2022).
Panthee, S. et al. Complete genome sequence and comparative genomic analysis of Enterococcus faecalis EF-2001, a probiotic bacterium. Genomics 113, 1534–1542. https://doi.org/10.1016/j.ygeno.2021.03.021 (2021).
Takahashi, K. et al. Antidepressant effects of Enterococcus faecalis 2001 through the regulation of prefrontal cortical myelination via the enhancement of CREB/BDNF and NF-kappaB p65/LIF/STAT3 pathways in olfactory bulbectomized mice. J. Psychiatr. Res. 148, 137–148. https://doi.org/10.1016/j.jpsychires.2022.01.047 (2022).
Takahashi, K. et al. Antidementia effects of Enterococcus faecalis 2001 are associated with enhancement of hippocampal neurogenesis via the ERK-CREB-BDNF pathway in olfactory bulbectomized mice. Physiol. Behav. 223, 112997. https://doi.org/10.1016/j.physbeh.2020.112997 (2020).
Belmer, A., Patkar, O. L., Lanoue, V. & Bartlett, S. E. 5-HT1A receptor-dependent modulation of emotional and neurogenic deficits elicited by prolonged consumption of alcohol. Sci. Rep. 8, 2099. https://doi.org/10.1038/s41598-018-20504-z (2018).
Nyuyki, K. D., Cluny, N. L., Swain, M. G., Sharkey, K. A. & Pittman, Q. J. Altered brain excitability and increased anxiety in mice with experimental colitis: Consideration of hyperalgesia and sex differences. Front. Behav. Neurosci. 12, 58. https://doi.org/10.3389/fnbeh.2018.00058 (2018).
Cooper, H. S., Murthy, S. N., Shah, R. S. & Sedergran, D. J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Investig. 69, 238–249 (1993).
Vasina, V. et al. Non-peptidyl low molecular weight radical scavenger IAC attenuates DSS-induced colitis in rats. World J. Gastroenterol. 16, 3642–3650. https://doi.org/10.3748/wjg.v16.i29.3642 (2010).
Kimijima, H. et al. Trichostatin A, a histone deacetylase inhibitor, alleviates the emotional abnormality induced by maladaptation to stress in mice. Neurosci. Lett. 766, 136340. https://doi.org/10.1016/j.neulet.2021.136340 (2022).
Kurokawa, K. et al. Activation of 5-HT(1A) receptor reduces abnormal emotionality in stress-maladaptive mice by alleviating decreased myelin protein in the ventral hippocampus. Neurochem. Int. 151, 105213. https://doi.org/10.1016/j.neuint.2021.105213 (2021).
Kurokawa, K. et al. Leukemia inhibitory factor participates in the formation of stress adaptation via hippocampal myelination in mice. Neuroscience 446, 1–13. https://doi.org/10.1016/j.neuroscience.2020.08.030 (2020).
Takeda, H., Tsuji, M. & Matsumiya, T. Changes in head-dipping behavior in the hole-board test reflect the anxiogenic and/or anxiolytic state in mice. Eur. J. Pharmacol. 350, 21–29. https://doi.org/10.1016/s0014-2999(98)00223-4 (1998).
Paxinos, G. & Franklin, K. B. J. The Mouse Brain in Stereotaxic Coordinates (Academic Press, 2001).
Takahashi, K. et al. Antidepressant-like effect of aripiprazole via 5-HT1A, D1, and D2 receptors in the prefrontal cortex of olfactory bulbectomized mice. J. Pharmacol. Sci. 137, 241–247. https://doi.org/10.1016/j.jphs.2018.06.006 (2018).
Takahashi, K. et al. Dopamine D2 receptor supersensitivity in the hypothalamus of olfactory bulbectomized mice. Brain Res. 1746, 147015. https://doi.org/10.1016/j.brainres.2020.147015 (2020).
Takahashi, K. et al. Disturbance of prefrontal cortical myelination in olfactory bulbectomized mice is associated with depressive-like behavior. Neurochem. Int. 148, 105112. https://doi.org/10.1016/j.neuint.2021.105112 (2021).
Takahashi, K. et al. Brexpiprazole prevents colitis-induced depressive-like behavior through myelination in the prefrontal cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry 121, 110666. https://doi.org/10.1016/j.pnpbp.2022.110666 (2023).
Takahashi, K. et al. Correlation between the reduction in hippocampal SirT2 expression and depressive-like behaviors and neurological abnormalities in olfactory bulbectomized mice. Neurosci. Res. 182, 76–80. https://doi.org/10.1016/j.neures.2022.06.001 (2022).
Takahashi, M. et al. The effects of acute treatment with tandospirone, diazepam, and placebo on driving performance and cognitive function in healthy volunteers. Hum. Psychopharmacol. 25, 260–267. https://doi.org/10.1002/hup.1105 (2010).
Nicolini, C. & Fahnestock, M. The valproic acid-induced rodent model of autism. Exp. Neurol. 299, 217–227. https://doi.org/10.1016/j.expneurol.2017.04.017 (2018).
Uys, J. D., Stein, D. J., Daniels, W. M. & Harvey, B. H. Animal models of anxiety disorders. Curr. Psychiatry Rep. 5, 274–281. https://doi.org/10.1007/s11920-003-0056-7 (2003).
Sarkar, D. A review of behavioral tests to evaluate different types of anxiety and anti-anxiety effects. Clin. Psychopharmacol. Neurosci. 18, 341–351. https://doi.org/10.9758/cpn.2020.18.3.341 (2020).
Karl, T., Pabst, R. & von Hörsten, S. Behavioral phenotyping of mice in pharmacological and toxicological research. Exp. Toxicol. Pathol. 55, 69–83. https://doi.org/10.1078/0940-2993-00301 (2003).
Qin, X. et al. GABA(A)(δ) receptor hypofunction in the amygdala-hippocampal circuit underlies stress-induced anxiety. Sci. Bull. (Beijing) 67, 97–110. https://doi.org/10.1016/j.scib.2021.09.007 (2022).
McHugh, S. B., Deacon, R. M., Rawlins, J. N. & Bannerman, D. M. Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behav. Neurosci. 118, 63–78. https://doi.org/10.1037/0735-7044.118.1.63 (2004).
Aksu, I. et al. Maternal treadmill exercise during pregnancy decreases anxiety and increases prefrontal cortex VEGF and BDNF levels of rat pups in early and late periods of life. Neurosci. Lett. 516, 221–225. https://doi.org/10.1016/j.neulet.2012.03.091 (2012).
Alò, R. et al. Correlation of distinct behaviors to the modified expression of cerebral Shank 1,3 and BDNF in two autistic animal models. Behav. Brain Res. 404, 113165. https://doi.org/10.1016/j.bbr.2021.113165 (2021).
Reichmann, F., Painsipp, E. & Holzer, P. Environmental enrichment and gut inflammation modify stress-induced c-Fos expression in the mouse corticolimbic system. PLoS One 8, e54811. https://doi.org/10.1371/journal.pone.0054811 (2013).
Xiao, Q., Xu, X. & Tu, J. Chronic optogenetic manipulation of basolateral amygdala astrocytes rescues stress-induced anxiety. Biochem. Biophys. Res. Commun. 533, 657–664. https://doi.org/10.1016/j.bbrc.2020.09.106 (2020).
Zahra, A. et al. Prenatal exposure of citalopram elicits depression-like and anxiety-like behaviors and alteration of morphology and protein expression of medial prefrontal cortex in young adult mice. J. Integr. Neurosci. 21, 61. https://doi.org/10.31083/j.jin2102061 (2022).
Zhao, T. T. et al. Effects of (–)-sesamin on chronic stress-induced anxiety disorders in mice. Neurochem. Res. 42, 1123–1129. https://doi.org/10.1007/s11064-016-2146-z (2017).
Liu, Y., Sanderson, D., Mian, M. F., McVey Neufeld, K. A. & Forsythe, P. Loss of vagal integrity disrupts immune components of the microbiota–gut–brain axis and inhibits the effect of Lactobacillus rhamnosus on behavior and the corticosterone stress response. Neuropharmacology 195, 108682. https://doi.org/10.1016/j.neuropharm.2021.108682 (2021).
Acknowledgements
The authors would like to thank Ms. Momoko Sakima, Ms. Ayaka Saito, Ms. Sayaka Endo, and Ms. Ayaka Katano of the International University of Health and Welfare (Ohtawara, Japan) for their technical assistance.
Funding
This study was supported, in part, by JSPS KAKENHI [Grant number JP21K15351].
Author information
Authors and Affiliations
Contributions
K.T.: Validation, methodology, conceptualization, writing—original draft, formal analysis, funding acquisition; M.T.: Writing—review & editing; O.N.: Writing—review & editing, methodology, investigation; K.M.: Investigation; K.K.: Investigation; A.M.-S.: Investigation; H.T.: Supervision; M.I.: Supervision; H.I.: Supervision; S.S.: Supervision; T.T.: Conceptualization, supervision, writing—review & editing, project administration. All authors read and approved this paper.
Corresponding author
Ethics declarations
Competing interests
Masahiro Iwasa, Hiroyuki Iwasa, and Shigeo Suzuki are employees of Nihon Berm Co., Ltd. All other authors declare that they have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Takahashi, K., Tsuji, M., Nakagawasai, O. et al. Anxiolytic effects of Enterococcus faecalis 2001 on a mouse model of colitis. Sci Rep 14, 11519 (2024). https://doi.org/10.1038/s41598-024-62309-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-62309-3
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.