Abstract
Delays in processing are frequent because of problems associated with transporting the samples to the laboratory. Thus, we aimed to evaluate the effect of sample storage on the Peroxidation of Leukocytes Index Ratio (PLIR). Differences between PLIR values of lymphocytes (PLIR-L), monocytes (PLIR-M) and granulocytes (PLIR-G) were observed in fresh samples. Sample storage affected the evaluation of PLIR. In particular, PLIR-L was lower in stored samples compared to fresh samples. In conclusion, our results suggest that fresh samples are recommended for assessing the PLIR.
Similar content being viewed by others
Introduction
The oxidation products of lipids, DNA and proteins and/or the enzymatic and non enzymatic antioxidants are usually measured in order to evaluate the oxidative stress, associated with many pathological and sub-clinical conditions1. However, the major bias of the total antioxidant capacity methods is that they are strongly influenced by the presence of products of catabolism, such as bilirubin and uric acid2,3. Furthermore, the FRAP method (Ferric Reducing Antioxidant Potential) matches the antioxidant capacity to the reducing ability3. It is well known that reduced iron is critical in the onset of oxidative stress due to the Fenton reaction, that generates the hydroxyl radical initiator of lipid peroxidation1. On the other hand, the role of uric acid and bilirubin in the prognosis of oxidative stress-related diseases is still controversial4,5.
From the computational analysis of markers of oxidation (plasma malondialdehyde, oxidized glutathione and urinary isoprostanes) and antioxidants (reduced glutathione, tocopherol and plasma antioxidant capacity) an index of global oxidative stress (OXY-SCORE) has been proposed6. However, this type of approach pictures only the redox state without considering the important role of free radicals in the innate response (NADPH-oxidase, myeloperoxidase) and in the resistance to infection, that declines during aging7.
The Peroxidation of Leukocytes Index Ratio (PLIR)8 is a test that measures both the resistance of leukocytes to an exogenous oxidative stress and the leukocytes functional capacity of oxidative burst upon activation. Furthermore, unlike the intracellular probes, PLIR is not affected by the interference of substrates of the multidrug resistance proteins9. Therefore, PLIR could be a flexible method to study the redox balance in both preclinical and clinical conditions, as well as to evaluate the effects of pharmacological or nutritional interventions. However, delays in processing are frequent because of problems associated with transporting the samples to the laboratory.
Therefore, we aimed to evaluate the effect of sample storage on the PLIR method.
Results and Discussion
After the exclusion of dead cells and/or debris (D) on forward (FSC) versus (vs.) side scatter (SSC) plots, lymphocytes (L), monocytes (M) and granulocytes (G) were selected by CD45-APC vs. SSC plots (Fig. 1).
The fluorescent probe 4,4-difluoro-5-(4-phenyl-1, 3-butadienyl)-4-bora-3a, 4a-diaza-s-indacene-3-undecanoic acid (C11-BODIPY), used in the PLIR method, modifies its fluorescence from red (FL2) to green (FL1)8,10 as a result of oxidation. We observed both the expected increase in FL1 and decrease in FL2 upon oxidation, contrarily to the unexpected increase of C11-BODIPY fluorescence at 600 nm observed by measuring the progression of neutrophil membrane oxidation upon activation by monitoring the decay of red fluorescence11.
The oxidized/reduced fluorescence ratio of C11-BODIPY has been used in order to normalize for cell incorporation of the probe into membrane8. We derived the RATIO (FL1/FL2) by FlowJo software. Treatment with 2,2′-azobis(2-methylpropionamidine) dihydrochloride (AAPH) or phorbol 12-myristate 13-acetate (PMA) treatment increased the RATIO of fluorescence, but in a different manner, showing that oxidative burst induced reactive oxygen species (ROS) production only in activated cells, while all cells were sensitive to exogenous ROS injury (Fig. 1). The vitamin E analogue 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), did not affect baseline level of oxidation and inhibited the peroxidation of C11-BODIPY in leukocytes exposed to AAPH free radicals generating system. The AAPH-induced oxidation was greater in fresh samples than in 4–8°C stored samples (Fig. 1). From the gated populations on CD45-APC vs. SSC plots (Fig. 1), we calculated the PLIR-L, PLIR-M and PLIR-G as previously described8.
Statistical analysis, carried out with Friedman Repeated Measures Analysis of Variance (RM ANOVA) on Ranks, revealed a normal distribution for L, M and G populations (Normality Test Shapiro-Wilk passed: L: p = 0,308; M: p = 0,386; G: p = 0,326).
One Way RM ANOVA, with type of sample as within-subjects factor, revealed that the differences in the mean values among the sample groups were greater than would be expected by chance for L (P = 0.002; Power of performed test with alpha = 0.050: 0.916) and M (p = 0,004; Power of performed test with alpha = 0.050: 0.839). To isolate the group that differs from the others we used the Bonferroni post-hoc analysis (All Pairwise Multiple Comparison Procedure).
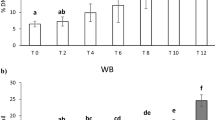
Decreases in PLIR-L (fresh vs. 4–8°C: p = 0.002; fresh vs. 18–22°C: p = 0.026; power of performed test with alpha = 0.050: 0.916) and PLIR-M (fresh vs. 4–8°C: p = 0.004; power of performed test with alpha = 0.050: 0.839) were observed in the stored samples compared to fresh samples (Fig. 2).
Raw data of PLIR values of lymphocytes (L), monocytes (M) and granulocytes (G), measured in fresh samples or samples stored at 18–22°C or 4–8°C for 24 h (A). Comparisons for factor sample within L: †p < 0.05, ‡p < 0.01; within M: §p < 0.01. Comparisons for factor cell within sample: L versus G or M **p < 0.01, ***p < 0.001. Typical dot plots Derived (FL1/FL2) versus SSC of L, M and G from samples stored at 4–8°C for 24 h, un-stimulated (UNST) (B) or treated with Trolox (10 μM) (C), AAPH (10 mM) (D) or PMA (1 μg/ml) (E). D: dead cells and/or debris.
In order to investigate the possible interaction between sample storage and cell type, we performed two way RM ANOVA, with sample or cell type as within-subjects factors (Equal Variance Test passed: p = 0.826; power of performed test with alpha = 0.05 for sample: 0.875, for cell: 0.999, for sample × cell: 0.645). There was a significant interaction between sample and cell (P = 0.016). In order to isolate differences between groups, the Bonferroni post-hoc analysis was used.
In fresh samples PLIR-L was significantly different compared to PLIR-M (p < 0.001) and to PLIR-G (p < 0.001) (Fig. 2).
The differences between the PLIR values of different populations were attenuated or lost after storage at 18–22°C (PLIR-L vs. PLIR-G p = 0.002; PLIR-L vs. PLIR-M p = 0.03) or 4–8°C, respectively (Fig. 2).
These results could be due to the high percentage of D events observed in the 4–8°C stored samples (Fig. 2). In particular, a population, resembling the apoptotic eosinophils described by Sandström et al.12, showed neither the increase in the RATIO of fluorescence nor the reduction of SSC after PMA-activation (Fig. 2). On the other hand, after AAPH treatment an increase in the oxidation was seen in D events (Fig. 2). Therefore, despite D events had been excluded from the PLIR analysis, they could scavenge cells from free radicals generated by AAPH and their presence in the reaction tube could interfere with the oxidation of the other cells.
On the other hand, the AAPH-induced oxidation in the 4–8°C stored samples could be due to changes in the antioxidant capacity after storage. In particular, it has been reported that the quality of stored blood can be deteriorated by haemolysis caused by free radicals. Markers of haemolysis and free radical metabolism have been previously examined in samples with high (HL) and low (LL) leukocyte count13. Authors observed an increase in the antioxidant capacity in blood samples stored at 4°C, which was accompanied by haemolysis13. In post-storage samples, the increase of the lipoperoxidation marker malondialdehyde was significantly higher in HL samples, suggesting that leukocytes participate in free radical production in stored blood. Besides, it is known that bilirubin, the product of haemoglobin catabolism, increases the antioxidant capacity2,3,4, whereas iron is involved in the generation of the lipid peroxidation1. Therefore, haemolysis during storage could affect the PLIR evaluation. These considerations could also explain the Trolox-induced increases in the percentage of D events in some of the PMA-activated samples stored at 4–8°C (Fig. 2). This effect was probably due to the iron reducing activity3, which could increase the Fenton reaction subsequent to hydrogen peroxide production induced by oxidative burst. On the other hand, a less pronounced protection on AAPH-induced oxidation has been observed, probably due to the loss of the known synergisms between antioxidants2. In support of this hypothesis, plasma antioxidant capacity it is known to decrease with storage2.
Overall these results suggest that PLIR is sensitive to the concentration of plasma antioxidants, as well as to the products of catabolism and iron generated by haemolysis.
Interestingly, on fresh samples we found PLIR ranges (PLIR-L Range 1.70–4.92; PLIR-M Range 1.53–4.07; PLIR-G Range 1.54–3.83) larger than those previously reported (PLIR-L Range 0.69–1.64; PLIR-M Range 0.84–1.26; PLIR-N Range 0.84–1.15)8. It is possible that the filters of the flow cytometer Accuri BD (FL1 533/30 nm; FL2 585/40 nm) are better centred on the two fluorescence of C11-BODIPY, compared to those of the flow cytometer Epics XL Coulter (FL1 525/20 nm; FL2 575/25 nm; FL3 620/15 nm), thus increasing the sensitivity14,15. Further studies are needed to evaluate the best filter tool to measure PLIR.
In conclusion, we have observed a significant difference between PLIR of different leukocytes subset on fresh samples. Such differences were attenuated after storage of the samples. Therefore, our results suggest that fresh samples are recommended for assessing the PLIR.
Methods
This work can be considered an observational and/or a quality control/improvement project. Furthermore, taking a single blood sample from a healthy volunteer would generally only present a minimal risk and might therefore be regarded as acceptable. Therefore no Regional Ethics Committee approval was required16, but all procedures involving human subjects complied with the Declaration of Helsinki as revised in 2000. Written informed consent was obtained from all the participants in accordance with Italian law (law n° 196/2003; Ministry of Health Circular Letter GU n° 76/2008).
Venous peripheral blood, from 14 healthy volunteers (10 men and 4 women, aged between 26 and 35 years), was collected after an overnight fasting. PLIR has been evaluated immediately after the collection and after 24 hours of storage at 4–8°C or at 18–22°C. After red blood cells' lysis and staining with C11-BODIPY (cod. D3861, lot. 1381315, Invitrogen Molecular Probes, final concentration 1 μM), leukocytes were treated as previously described8 with PMA (cod. P1585, lot. SLBC7913V, Sigma, final concentration 1 μg/ml), AAPH (cod. 440914, lot. MKBL5129V, Sigma-ALDRICH, final concentration 10 mM), Trolox (cod. 238813, lot. BCBM7406VP, Sigma-ALDRICH, final concentration 10 μM), PMA 1 μg/ml + Trolox 10 μM or AAPH 10 mM + Trolox 10 μM. After 30 min at 37°C cells were stored in ice, to stop reaction and rapidly analyzed on an Accuri C6 BD cytometer. Cell surface staining of CD45 was performed with the anti-CD45-APC (clone HI30, isotype IgG1, κ, cod. 555485, lot. 3291614, BD Pharmingen).
Fluidic calibration of the Accuri C6 BD cytometer was performed before each experiment, as suggested by the manufacturer. Threshold was set at the FS parameter and samples were acquired until 10000 events were collected in the gate of CD45 positive cells.
Data acquired on the Accuri C6 was exported in FCS format and analyzed by FlowJo software to calculate the fluorescence ratio (FL1/FL2) as derived parameter. The PLIR was calculated, as previously described8, from the formula:

All statistical evaluations were performed using the SigmaStat and Sigmaplot software (Jandel Scientific, Inc.).
References
Knasmuller, S. et al. Use of conventional and -omics based methods for health claims of dietary antioxidants: a critical overview. Br. J. Nutr. 99, ES3–ES52 (2008).
Ghiselli, A., Serafini, M., Maiani, G., Azzini, E. & Ferro-Luzzi, A. A fluorescence-based method for measuring total plasma antioxidant capability. Free Radic. Biol. Med. 18, 29–36 (1995).
Benzie, I. F. F. & Strain, J. J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal. Biochem. 239, 70–76 (1996).
Basiglio, C. L. et al. Complement activation and disease: protective effects of hyperbilirubinaemia. Clin. Sci. (Lond). 118, 99–113 (2009).
Alvarez-Lario, B. & Macarrón-Vicente, J. Is there anything good in uric acid? QJM. 104, 1015–1024 (2011).
Veglia, F. et al. Age- and gender-related oxidative status determined in healthy subjects by means of OXY-SCORE, a potential new comprehensive index. Biomarkers. 11, 562–573 (2006).
Magrone, T. & Jirillo, E. Mechanisms of neutrophil-mediated disease: innovative therapeutic interventions. Curr. Pharm. Des. 18, 1609–1619 (2012).
Peluso, I. et al. A new flow cytometry method to measure oxidative status: the Peroxidation of Leukocytes Index Ratio (PLIR). J. Immunol. Methods. 390, 113–120 (2013).
Peluso, I., Manafikhi, H., Reggi, R. & Palmery, M. Interference of flavonoids with fluorescent intracellular probes: Methodological implications in the evaluation of the oxidative burst by flow cytometry. Cytometry A. 85, 663–77 (2014).
BODIPY® 581/591 C11 (Lipid Peroxidation Sensor) (Molecular Probes®) http://www.lifetechnologies.com/order/catalog/product/D3861 (Date of access:30/05/2014).
de Souza-Junior, T. P. et al. Supra-physiological doses of testosterone affect membrane oxidation of human neutrophils monitored by the fluorescent probe C11-BODIPY581/591. Eur. J. Appl. Physiol. 113, 1241–1248 (2013).
Sandström, K., Håkansson, L., Lukinius, A. & Venge, P. A method to study apoptosis in eosinophils by flow cytometry. J. Immunol. Methods. 240, 55–68 (2000).
Racek, J., Herynková, R., Holecek, V., Faltysová, J. & Krejcová, I. What is the source of free radicals causing hemolysis in stored blood? Physiol. Res. 50, 383–388 (2001).
Fluorophore selection guide for flow cytometry Dye choices for your instrument made easy. (Invitrogen). http://www.pasteur.fr/ip/portal/action/WebdriveActionEvent/oid/01s-00002m-02o/fname/O-072942_FlowInstrGuide_final2%20(2).pdf (Date of access:30/05/2014).
Fluorescence SpectraViewer (http://www.lifetechnologies.com/it/en/home/life-science/cell-analysis/labeling-chemistry/fluorescence-spectraviewer.html (Date of access:30/05/2014).
Rosmini, F. & Scuderi, G. Consulting an ethics committee, when is it necessary? Two examples. Epidemiol. Prev. 29, 210–13 (2005).
Acknowledgements
We thank Claudio Andrew Gobbi and Juliet Donna Qadir for English review of the manuscript.
Author information
Authors and Affiliations
Contributions
I.P. designed the research, analyzed the data and drafted the manuscript. H.M. performed the analyses. C.Z. assessed the healthy status of the subject and performed the blood sampling. F.A. critical reviewed the manuscript. M.P. supervised the whole project.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Peluso, I., Manafikhi, H., Altieri, F. et al. The effect of sample storage on the Peroxidation of Leukocytes Index Ratio (PLIR) measure. Sci Rep 4, 6539 (2014). https://doi.org/10.1038/srep06539
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep06539
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.