Abstract
Materials with zero/near zero thermal expansion coefficients are technologically important for applications in thermal management and engineering. To date, this class of materials can only be produced by chemical routes, either by changing chemical compositions or by composting materials with positive and negative thermal expansion. Here, we report for the first time a physical route to achieve near zero thermal expansion through application of pressure. In the stability field of tetragonal PbTiO3 we observed pressure-induced reversals between thermal contraction and expansion between ambient pressure and 0.9 GPa. This hybrid behavior leads to a mathematically infinite number of crossover points in the pressure-volume-temperature space and near-zero thermal expansion coefficients comparable to or even smaller than those attained by chemical routes. The observed pressures for this unusual phenomenon are within a small range of 0.1–0.9 GPa, potentially feasible for designing stress-engineered materials, such as thin films and nano-crystals, for thermal management applications.
Similar content being viewed by others
Introduction
Most materials expand on heating and contract on cooling. Although uncommon, some materials exhibit opposite behavior and possess negative coefficients of thermal expansion (CTE). There is yet a third family of materials that neither expand nor contract as temperature changes and they display zero thermal expansion (ZTE) within a certain temperature range1,2,3,4,5,6. ZTE is an intriguing and useful physical attribute, because materials with ZTE do not undergo thermal shock or fatigue during rapid or repeated heating and cooling cycles, which makes them excellent candidates for applications in optics, electronics and heat-engine components. ZTE is typically achieved by composting materials with positive and negative thermal expansion (NTE), but this route is often hindered by poor thermal stability of materials with NTE7. The mismatch in thermal expansion also results in internal strains in composite materials at elevated temperatures. Chemical substitution, operating as “chemical stress”, is another common method to modify structural and physical properties of materials8,9. In ABO3 perovskite, for example, chemical substitutions over both the A and B sites are effective pathways to tune materials with NTE or even ZTE over a large temperature range, as demonstrated by the studies in PbTiO3-based pervskites10,11,12,13,14,15. ZTE phenomenon is also fundamentally interesting because it is associated with rich and complex physical mechanisms1,2,4,5,6, such as Invar effect, valence-state transition, strain relaxation of the constituent polyhedra in crystal structures and elastic anisotropy.
Lead titanate, PbTiO3, is one of the most widely used ferroelectric and piezoelectric materials in industry16,17,18. At ambient conditions, PbTiO3 possesses a tetragonal structure (P4mm) with an axial ratio of c/a = 1.0619. The structural distortion from the ideal, cubic structure (space group Pm3m) is generally attributed to the covalent nature of B-O bonds in ABO3-type ferroelectric perovskite. In tetragonal PbTiO3, the 6s2 lone pairs effect of Pb2+ and the Pb-O hybridization are not only responsible for the extra degree of structural distortion20,21, high spontaneous polarization and relatively high Curie temperature (763 K)22, they are also important mechanisms underlying the observed NTE10,11,12,13,14,23. The mean CTE of this compound is −1.99×10−5 K−1 over the temperature range of 300–763 K15 and the corresponding thermal contraction is closely related to the ferroelectric soft modes with increasing temperature15. Above 763 K, the tetragonal PbTiO3 transforms to a paraelectric cubic phase that exhibits the regular positive CTE17,24,25,26,27,28.
As an important thermodynamic variable, pressure can effectively change the bonding characteristics (bond lengths and angles) and electron clouds overlapping of the constituent atoms in a compound, which would ultimately alter the material's structural and physical properties. In PbTiO3, both tetragonality and ferroelectricity are progressively suppressed with increasing pressure at 300 K, leading to a paraelectric cubic phase at pressures above 12 GPa29. Compared with chemical substitution, the application of pressure is a straightforward route to tune material properties without introducing impurities and/or vacancies into the parent structure. Such defects are known to affect many properties such as structural stability, chemical diffusion, electric and ionic conductivities and elasticity. In the present work, we applied external pressure to manipulate CTE of tetragonal PbTiO3, including the achievement of near zero thermal expansion. This approach was motivated by our earlier findings30 that at pressures above 1 GPa PbTiO3 expands at all temperatures within the stability field of tetragonal phase. This behavior implies a crossover in the volumetric thermal expansion from negative to positive between ambient pressure and 1 GPa, which would presumably lead to mean ZTE over certain temperature ranges. Such pressure-tuned ZTE, if it can experimentally be verified, is not only scientifically important but also technologically relevant because similar levels of stress can readily be realized in thin-film and nanocrystalline PbTiO3 produced by stress engineering31,32,33.
Results
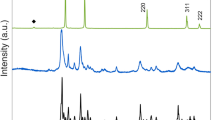
Figure 1 shows a representative neutron diffraction pattern of PbTiO3 analyzed by the Rietveld method. The refined structural parameters, such as lattice parameters, atomic positions, goodness of fit and R factors, are listed in Table 1. Figure 2a shows the pressure dependence of unit-cell volumes of tetragonal PbTiO3 at different temperatures. In striking contrast to the compression behavior in most materials, we observe a number of crossovers between the P-V isotherms within the 0–1 GPa range (Fig. 2a), implying reversals in the sign of CTE of PbTiO3. At 0.1 GPa, there is a crossover point between the 300 K and 480 K volume isotherms, indicating that the mean volumetric CTE,  , is zero over this temperature range. Similar to the behavior at ambient pressure, tetragonal PbTiO3 shrinks at temperatures above 480 K with a negative CTE. The second crossover point is located at ~0.2 GPa between the 300 K and 580 K isotherms. PbTiO3, however, expands with increasing temperature between 300 and 480 K and continues to contract at higher temperatures. The third crossover point is found at ~0.55 GPa between the 300 and 730 K isotherms. At this pressure, the structure expands between 300 and 580 K with essentially zero thermal expansion between 480 and 580 K isotherms; the
, is zero over this temperature range. Similar to the behavior at ambient pressure, tetragonal PbTiO3 shrinks at temperatures above 480 K with a negative CTE. The second crossover point is located at ~0.2 GPa between the 300 K and 580 K isotherms. PbTiO3, however, expands with increasing temperature between 300 and 480 K and continues to contract at higher temperatures. The third crossover point is found at ~0.55 GPa between the 300 and 730 K isotherms. At this pressure, the structure expands between 300 and 580 K with essentially zero thermal expansion between 480 and 580 K isotherms; the  is zero over the 300–730 K range due to thermal contraction between 580 and 730 K. Evidently, at a given pressure, tetragonal PbTiO3 exhibits a hybrid behavior of contraction and expansion. Such unusual behavior eventually leads to three additional crossovers at higher pressures, 0.65 GPa between 480 K and 580 K isotherms, 0.85 GPa between 480 K and 730 K isotherms and 0.9 GPa between 580 K and 730 K isotherms. When pressure is above 0.9 GPa, the unit-cell volumes expand at all experimental temperatures within the stability field of tetragonal PbTiO3, which is consistent with our earlier observations using synchrotron XRD30.
is zero over the 300–730 K range due to thermal contraction between 580 and 730 K. Evidently, at a given pressure, tetragonal PbTiO3 exhibits a hybrid behavior of contraction and expansion. Such unusual behavior eventually leads to three additional crossovers at higher pressures, 0.65 GPa between 480 K and 580 K isotherms, 0.85 GPa between 480 K and 730 K isotherms and 0.9 GPa between 580 K and 730 K isotherms. When pressure is above 0.9 GPa, the unit-cell volumes expand at all experimental temperatures within the stability field of tetragonal PbTiO3, which is consistent with our earlier observations using synchrotron XRD30.
(a) The pressure dependence of unit cell volumes of tetragonal PbTiO3 at different temperatures. (2b), the lattice volumes of tetragonal PbTiO3 as a function of temperature at constant pressures. The solid curves at 0.3 GPa, 0.8 GPa and 1.2 GPa are the data interpolated from Figure 2a. The plotted symbols are the experimental data points collected within ±0.1 GPa of the indicated pressures. The pink shadow area denotes the region of thermal contraction and the rest plain area corresponds to the region of thermal expansion. The ΔTEi represents the temperature range for expansion and ΔTCi the temperature range for contraction. The subscripts of 1, 2, 3 and 4 in ΔTEi and ΔTCi correspond to the pressures of 0 GPa, 0.3 GPa, 0.8 GPa and 1.2 GPa, respectively. The turnover points from expansion to contraction are 447 K at 0.3 GPa and 556 K at 0.8 GPa.
As a result of pressure-induced reversal between thermal contraction and expansion, there are mathematically an infinite number of crossover points in the P-V-T space, giving the infinitesimal ΔTs between two volume isotherms, where ΔT = T2 – T1 with T2 > T1. Our findings indicate that the larger the mean temperature of T1 and T2, the higher the pressure is needed to produce the crossover between the corresponding isotherms (Fig. 2a). This behavior would in principle lead to mean ZTE coefficients over an infinite number of ΔTs. The exact temperature range for ZTE or near ZTE, however, cannot be determined based on the measured data because pressure varies with heating in complicated manners that depend on thermal pressure, deviatoric stress and materials' strength. To determine the temperature ranges for ZTE, we interpolated the experimental data of Figure 2a and plot the lattice volumes as a function of temperature in Figure 2b at three constant pressures of 0.3, 0.8 and 1.2 GPa, arbitrarily chosen for clarity. Strictly speaking, there are no such temperature ranges in which PbTiO3 exhibits truly zero thermal expansion (except at the “turnover” points of 447 and 556 K where CTE changes from positive to negative). In other words, CTEs would deviate from ZTE when the temperature is away from the “turnover” points. However, pressure plays a significant role in changing thermal behavior from contraction at ambient pressure to expansion above 0.9 GPa. In addition, pressure tends to elevate the temperature of turnover point, which leads to increased temperature ranges for the expansion (ΔTEi) and reduced ranges for the contraction (ΔTCi) as pressure increases. Consequently, within the 150 K range of the turnover points, the lattice volumes of PbTiO3 are nearly independent of temperature. Based on such interpolated data, we calculated  values at pressures of 0.3 GPa and 0.8 GPa, which are listed in table 2 along with those previously obtained from chemical substitutions for PbTiO3-based perovskite. Strictly speaking, neither chemical nor pressure-tuned route produces thermal expansion coefficients that are truly zero. Nevertheless, the resultant CTEs are substantially smaller than those for chemically pure PbTiO3 at ambient pressure. At both pressures, the pressure-tuned “near-zero” CTEs are comparable to or even smaller than those attained by chemical routes, indicating that pressure-tuning can be at least as effective as chemical routes for controlling materials' thermal behavior.
values at pressures of 0.3 GPa and 0.8 GPa, which are listed in table 2 along with those previously obtained from chemical substitutions for PbTiO3-based perovskite. Strictly speaking, neither chemical nor pressure-tuned route produces thermal expansion coefficients that are truly zero. Nevertheless, the resultant CTEs are substantially smaller than those for chemically pure PbTiO3 at ambient pressure. At both pressures, the pressure-tuned “near-zero” CTEs are comparable to or even smaller than those attained by chemical routes, indicating that pressure-tuning can be at least as effective as chemical routes for controlling materials' thermal behavior.
(a) A Evolution of lattice parameters a and c of tetragonal PbTiO3 with increasing pressure at 300 K, 480 K, 580 K and 730 K. (3b), tetragonality (c/a) of PbTiO3 as functions of temperature and pressure. The solid lines are drawn from linear fitting and the determined pressure derivatives, d(c/a)/dP, are −0.013(2)/GPa, −0.0057(15)/GPa, −0.0039(16)/GPa and −0.00048(107)/GPa for 300 K, 480 K, 580 K and 730 K, respectively.
Discussion
In most materials, thermal expansion decreases with increasing pressure, which would in principle lead to a vanishing α at a finite pressure34. However, this general phenomenon does not allow the crossovers in pressure-volume isotherms. In fact, the extrapolation of experimental P-V-T data often requires some additional empirical constants to prevent such crossovers from occurring35. In the present work, we demonstrate for the first time that the P-V isotherms can crossover in materials with NTE at ambient pressure. The pressure-induced reversal from thermal contraction to expansion also represents a new physical route for tuning near-zero CTEs.
The pressure-tuned near-zero CTE in PbTiO3 is technologically relevant to applications in thermal management and engineering. The required pressures for these phenomena are in a small range of 0.1–0.9 GPa, which can readily be realized in thin films and nano-crystals manufactured by stress-engineering processes. It is well known that the residual stress in thin films can be manipulated by their thickness or mismatch with substrate materials. For PbTiO3 deposited on a Si(100) substrate31, the stresses in thin films of 407 nm and 92 nm thickness are 0.96 (9) GPa and 1.77(12) GPa, respectively. They can be increased to the 1.3–2.6 GPa range when PbTiO3 thin films of 400–50 nm thickness are deposited on a Pt-coated Si substrate32. It is well-known that in thin films the in-plane lattice parameters are constrained by the substrate materials. However, as a result of Pb-O and Ti-O bonding hybridizations, the a and c axis are coupled in the PbTiO3 thin film, as demonstrated in ref [36]. Therefore, the stress can still affect the out-of-plane lattice parameter and manipulate the CTEs of PbTiO3 thin films. Nano-synthesis can also introduce different levels of residual stress on the surface layer of a nanocrystal. The estimated stress in PbTiO3 nanocrystals of 15 nm can be as high as 0.9 GPa based on the comparison of c/a ratios between this work and Ref. [33]. Thus, the present findings not only offer a physical route to tune near-zero CTEs in chemically-pure PbTiO3 but may also potentially be useful for designing stress-engineered materials, such as thin films and nano-crystals, of desired thermal expansion coefficients, including ZTE. Last but not least, we expect that pressure-induced ZTE may occur in other NTE materials if they are structurally stable over the desired P-T space.
The thermal expansion behavior and associated ferroelectric polarization in PbTiO3 perovskite are commonly linked with its tetragonality and chemical bonding. To understand the correlation between these characteristics and the present findings in the pressure-temperature space unexplored by previous studies, we look into the details of c/a ratios and hybridization between Pb and O atoms. Plotted in Figure 3a are the lattice parameters as a function of pressure for tetragonal PbTiO3 at 300 K, 480 K, 580 K and 730 K. With increasing pressure, both lattice parameters a and c decrease (Figure 3a). However, the rate of decrease in c is considerably larger than that in a, leading to smaller c/a ratios at high pressures. Consequently, as the pressure increases, the tetragonal PbTiO3 approaches the cubic structure. Figure 3a also reveals the effect of temperature on the structural distortion; at a given pressure, lattice parameter a increases with increasing temperature whereas c decreases. In addition, the magnitudes of these variations decrease with increasing pressure. Figure 3b shows that the c/a ratios are suppressed by both temperature and pressure, indicating that pressure can effectively modulate the tetragonality of PbTiO3.
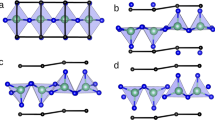
The thermal expansion behavior in tetragonal PbTiO3 under atmospheric pressure is commonly interpreted from the symmetry argument and Pb-O bonding hybridization37. The first one is based on the fact that both the PbO12 and TiO6 polyhedra become increasingly distorted as temperature decreases. The distortion would introduce the lattice strain21 and as the temperature is raised and the polyhedra become more regular toward the Curie point, the unit-cell volume contracts continuously in response to the lattice strain relaxation. The second mechanism is complementary to the first one with more focus on the hybridization between the Pb 6s and O 2p states. There are two distinct atomic positions for oxygen in the structure of tetragonal PbTiO3. Pb is strongly covalent with OII, while the Pb-OI bond shows ionicity. The longer Pb-OII bonds alternate with shorter Pb-OII bonds along the c axis, as shown in Figure 4a. It is well known that as the unequal bond lengths become equal, their average value decreases, which, in the case of PbTiO3, would lead to the volume contraction as the tetragonal phase approaches the tetragonal-cubic phase transition temperature.
(a) A schematic illustration of crystal structure for tetragonal PbTiO3 showing the alternative Pb-OII and Ti-OI bonds along the c axis. (4b), variation of short and long Pb-OII bond lengths as functions of temperature and pressure. Similar bond equalization tendency is also observed in Ti-OI bonds. The solid lines are drawn from linear fitting to guide eyes.
To gain insights into chemical bonding and associated thermal behavior at high pressure we refined the atomic positions in tetragonal PbTiO3 from the collected neutron diffraction data, as summarized in Table 1. Figure 4b shows the short and long Pb-OII bond lengths as functions of pressure and temperature. At all the temperatures the two Pb-OII bonds lengths are found to approach each other with increasing pressure, which is consistent with the prediction of density functional theory computations38. At any given pressure, Figure 4b reveals a similar tendency of bond equalization with increasing temperature. This is not unexpected because both heating at atmospheric pressure and compression at room temperature lead to the tetragonal-cubic phase transition. In addition, at all the temperatures, the average values of short and long Pb-OII bond lengths (as well as Ti-OI bonds) show similar trends of variation of c/a ratios under pressure (Figure 3b). The observed trends for the bond-length equalization are also supported by high-pressure Raman spectroscopic data29. It is known that the spontaneous polarization is proportional to the square root of (c/a - 1). In Raman spectra this is reflected by the softening of the E(1TO) and A1(1TO) symmetry modes29 with increasing pressure. By symmetry, the ions vibrate in the ab-basal plane in E-symmetry modes, whereas they vibrate parallel to the c-axis in A1 symmetry modes. The softening of the E(1TO) and A1(1TO) modes ultimately reflects the fact that pressure weakens the bond strength and force constants responsible for the vibration frequency, which is directly correlated to the changes in ion positions and decrease in hybridization. Thus, based on the combined observations of c/a ratios and equalization in Pb-OII bonds, the crossover behavior in volume isotherms is physically associated with tetragonality and hybridization between Pb and O atoms, which are both suppressed by pressure in tetragonal PbTiO3.
Methods
We conducted high-pressure/high-temperature neutron diffraction experiments on PbTiO3 using a 500-ton, toroidal anvil press (TAP-98) at the beamline of High-Pressure-Preferred-Orientation (HIPPO), Los Alamos Neutron Science Center (LANSCE). Time-of-flight neutron data were collected using detector banks at a fixed angle of 2θ = ±90°. A PbTiO3 powder sample was purchased from Alfa Aesar (99.9% metal based purity) and is phase-pure based on XRD. Two layers of PbTiO3 and NaCl powders was first compressed into a cylindrical pallet of 5.4 mm in diameter and 7 mm in length and was then loaded into a high-P-T ceramic cell assembly specially designed for TAP-98 (see Ref [39] for detail). NaCl served as the internal pressure standard and the Decker's equation of state (EOS) was used to calculate the pressure. Temperature was monitored by a W/5%Re-W26%Re thermocouple placed next to the sample and was stable within 25 K over a period of several hours of data acquisition time. To release the deviatoric stress built up during room-temperature compression on the polycrystalline sample, all high P-T neutron data were collected after the sample had been heated at 800 K for several minutes at each desired loading pressure. The high-temperature data at ambient pressure were collected with an ILL furnace; the sample was loaded into a vanadium can of 0.95 cm in diameter, which has low attenuation for neutrons and can hold temperatures up to 1500 K. The sample was heated under vacuum. The 144°-bank data obtained in ILL furnace and the 90°-bank data obtained in TAP-98 were used for determination of structural parameters of PbTiO3 using the Rietveld method with the General Structure Analysis System (GSAS)40.
References
Mohn, P. A century of zero expansion. Nature 400, 18–19 (1999).
Margadonna, S., Prassides, K. & Fitch, A. N. Zero Thermal Expansion in a Prussian Blue Analogue. J. Am. Chem. Soc. 126, 15390–15391 (2004).
Sleight, A. Materials science: Zero-expansion plan. Nature 425, 674–676 (2003).
Zhang, Y. et al. Zero thermal expansion in a nanostructured inorganic-organic hybrid crystal. Phys. Rev. Lett. 99, 215901 (2007).
Salvador, J. R. et al. Zero thermal expansion in YbGaGe due to an electronic valence transition. Nature 425, 702–705 (2003).
Xu, H. et al. Structural mechanisms underlying near-zero thermal expansion in β-eucryptite: A combined synchrotron x-ray and neutron Rietveld analysis. J. Mater. Res. 14, 3138–3151 (1999).
Mary, T. A. et al. Negative Thermal Expansion from 0.3 to 1050 Kelvin in ZrW2O8 . Science 272, 90–92 (1996).
Valant, M. & Davies, P. K. Crystal Chemistry and Dielectric Properties of Chemically Substituted (Bi1.5Zn1.0Nb1.5)O7 and Bi2(Zn2/3Nb4/3)O7 Pyrochlores. J. Am. Ceram. Soc. 83, 147–153 (2000).
Moreo, A., Yunoki, S. & Dagotto, E. Phase Separation Scenario for Manganese Oxides and Related Materials. Science 283, 2034–2040 (1999).
Xing, X. R. et al. Novel thermal expansion of lead titanate. Rare Metals 22, 294 (2003).
Chen, J. et al. Neutron diffraction studies of structure and increasing splitting of LO-TO phonons in Pb1−xCdxTiO3 . J. Appl. Phys. 100, 074106 (2006).
Chen, J. et al. Structure and negative thermal expansion in the PbTiO3–BiFeO3 system. Appl. Phys. Lett. 89, 101914 (2006).
Hu, P. H. et al. B-site Dopant Effect on the Thermal Expansion in the (1-x)PbTiO3-xBiMeO3 Solid Solution (Me = Fe, In, Sc). J. Am. Ceram. Soc. 94, 3600–3603 (2011).
Hu, P. H. et al. Thermal Expansion, Ferroelectric and Magnetic Properties in (1 -x)PbTiO3−xBi(Ni1/2Ti1/2)O3 . J. Am. Chem. Soc. 132, 1925–1928 (2010).
Chen, J. et al. Zero Thermal Expansion in PbTiO3-Based Perovskites. J. Am. Chem. Soc. 130, 1144–1145 (2008).
Scott, J. F. & Paz De Araujo, C. A. Ferroelectric Memories. Science 246, 1400–1405 (1989).
Sicron, N. et al. Nature of the ferroelectric phase transition in PbTiO3 . Phys. Rev. B 50, 13168–13180 (1994).
Scott, J. F. Applications of Modern Ferroelectrics. Science 315, 954–959 (2007).
Shirane, G. & Hoshino, S. On the Phase Transition in Lead Titanate. J. Phys. Soc. Jpn. 6, 265–270 (1951).
Kuroiwa, Y. et al. Evidence for Pb-O Covalency in Tetragonal PbTiO3 . Phys. Rev. Lett. 87, 217601 (2001).
Cohen, R. E. Origin of ferroelectricity in perovskite oxides. Nature (London) 358, 136–138 (1992).
Glazer, A. M. & Mabud, S. A. Powder profile refinement of lead zirconate titanate at several temperatures. II. Pure PbTiO3 . Acta Cryst. B 34, 1065–1070 (1978).
Kobayashi, J., Uesu, Y. & Sakemi, Y. X-ray and optical studies on phase transition of PbTiO3 at low temperatures. Phys. Rev. B 28, 3866–3872 (1983).
Burns, G. & Scott, B. A. Lattice Modes in Ferroelectric Perovskites: PbTiO3 . Phys. Rev. B 7, 3088–3101 (1973).
Fontana, M. D., Hidrissi, H. & Wojcik, K. Displacive to Order-Disorder Crossover in the Cubic-Tetragonal Phase Transition of PbTiO3 . Europhys. Lett. 11, 419–424 (1990).
Nelmes, R. J. et al. Order-disorder behaviour in the transition of PbTiO3 . Ferroelectrics 108, 165–170 (1990).
Ravel, B. et al. Order-disorder behavior in the phase transition of PbTiO3 . Ferroelectrics 164, 265–277 (1995).
García, A. & Vanderbilt, D. First-principles study of stability and vibrational properties of tetragonal PbTiO3 . Phys. Rev. B 54, 3817–3824 (1996).
Sanjurjo, J. A., López-Cruz, E. & Burns, G. High-pressure Raman study of zone-center phonons in PbTiO3 . Phys. Rev. B 28, 7260–7268 (1983).
Zhu, J. L. et al. Thermal equations of state and phase relation of PbTiO3: A high P-T synchrotron x-ray diffraction study. J. Appl. Phys. 110, 084103 (2011).
Valim, D. et al. Evaluating the residual stress in PbTiO3 thin films prepared by a polymeric chemical method. J. Phys. D: Appl. Phys. 37, 744–747 (2004).
Fu, D. et al. Thickness dependence of stress in lead titanate thin films deposited on Pt-coated Si. Appl. Phys. Lett. 77, 1532–1534 (2000).
Akdogan, E. K. et al. Size effects in PbTiO3 nanocrystals: Effect of particle size on spontaneous polarization and strains. J. Appl. Phys. 97, 084305 (2005).
Birch, F. Thermal expansion at high pressures. J. Geophys. Res. 73, 817–819 (1968).
Saxena, S. K. & Zhang, J. Thermochemical and pressure-volume-temperature systematics of data on solids, examples: tungsten and MgO. Phys. Chem. Mineral. 17, 45–51 (1990).
Janolin, P.-E. et al. Temperature evolution of the structural properties of monodomain ferroelectric thin film. Appl. Phys. Lett. 90, 192910 (2007).
Sleight, A. W. Compounds That Contract on Heating. Inorg. Chem. 37, 2854–2860 (1998).
Frantti, J. et al. The Factors Behind the Morphotropic Phase Boundary in Piezoelectric Perovskites. J. Phys. Chem. B 113, 7967–7972 (2009).
Zhao, Y. et al. High-pressure neutron diffraction studies at LANSCE. Appl. Phys. A 99, 585–599 (2010).
Larson, A. C. & Von Dreele, R. B. General Structure Analysis System (GSAS)., Los Alamos National Laboratory Report LAUR 86–748 (2004).
Acknowledgements
This work was supported by the laboratory-directed research and development (LDRD) program of Los Alamos National Laboratory, which is operated by Los Alamos National Security LLC under DOE Contract No. DE-AC52-06NA25396. The experimental work has benefited from the use of the Lujan Neutron Scattering Center at Los Alamos Neutron Science Center, which is funded by the U.S. Department of Energy's Office of Basic Energy Sciences. We also acknowledge the support from NSF & MOST of China through research projects.
Author information
Authors and Affiliations
Contributions
J.Z.Z., H.X., Y.Z. and C.J. conceived the work; J.L.Z. and J.Z.Z. conducted the experiments with the help of S.V.; J.L.Z. analyzed the data and drafted the manuscript; J.Z.Z., H.X., J.F. and Y.Z. helped edit the manuscript and also provided inputs for the Discussion section.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Zhu, J., Zhang, J., Xu, H. et al. Pressure-induced reversal between thermal contraction and expansion in ferroelectric PbTiO3. Sci Rep 4, 3700 (2014). https://doi.org/10.1038/srep03700
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep03700
This article is cited by
-
Perovskite ferroelectric tuned by thermal strain
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.