Abstract
During spring of 2009, a new influenza virus AH1N1 spread in the world causing acute respiratory illness and death, resulting in the first influenza pandemic since 1968. Blood levels of potentially-toxic and essential elements of 40 pneumonia and confirmed AH1N1 were evaluated against two different groups of controls, both not infected with the pandemic strain. Significant concentrations of potentially-toxic elements (lead, mercury, cadmium, chromium, arsenic) along with deficiency of selenium or increased Zn/Cu ratios characterized AH1N1 cases under study when evaluated versus controlled cases. Deficiency of selenium is progressively observed from controls I (influenza like illness) through controls II (pneumonia) and finally pneumonia -AH1N1 infected patients. Cases with blood Se levels greater than the recommended for an optimal cut-off to activate glutathione peroxidase (12.5 μg/dL) recovered from illness and survived. Evaluation of this essential element in critical pneumonia patients at the National Institutes is under evaluation as a clinical trial.
Similar content being viewed by others
Introduction
In April 2009, a novel swine-origin influenza A (H1N1) virus was identified in patients from Mexico and North America. The new virus spread around the world and the World Health Organization (WHO) declared a pandemic level of 61. Since then, several publications presenting clinical and epidemiological characteristics and discussing risk factors associated to the H1N1 patients in Mexico have been published2,3,4. Mexico documented the largest number of confirmed cases during the first and subsequent outbreaks as documented in literature5. In this work we first documented major potentially-toxic elements concentration in 40 cases of pneumonia and confirmed viral infection among hospitalized patients at the National Institute for Respiratory Diseases (INER) in Mexico. Key nutritional essential elements have been also documented in this analysis. Two control groups, negative for the pandemic strain, were selected among Mexico City population at INER and the National Institute of Nutrition (INCMNSZ) and compared versus toxicology and essential element profiles of AH1N1 patients, a group of young Mexicans, overall in good health, that presented acute respiratory illness through AH1N1 viral infection during pandemic year6. We discussed and correlated these levels with the occurrence and severity of the viral infection disease.
Results
Participants of the study
Participants are divided in two main groups: Cases and Controls (I and II). The first group is referred to 40 AH1N1 cases (pneumonia hospitalized cases with confirmed viral infection through RT-PCR assay). Sample collection for this group was done from October 1st to December 31st 2009 and from January 15th to April 15th 2010 during pandemic year-period6. The second group is subdivided as Controls I and II. The first group (Controls I) is referred to 30 pneumonia cases negative for the pandemic strain through RT-PCR assay. The second group is referred to 64 persons admitted, treated for ILI (Influenza like Illness) symptoms also negative for the specific virus strain (through RT-PCR assay). Criteria for selection as a “control case” was being a relative living with the patient at the time of the disease, exposed to the virus through direct contact with him/her for at least three days before his/her hospitalization. Controlled cases were selected for the present study accordingly to the period of our key AH1N1 samples were collected, i.e., from October 2009 throughout April 2010. Inclusionary and exclusionary criteria for selection of the control groups are summarized in Table 1.
Admission features of AH1N1 cases and controls
Cases. The start time of symptoms and admission to hospital ranged from 7–12 days (mean: 9 days). All patients had fever, temperatures higher than 38.5°C, cough, respiratory distress and other clinical features described for Mexico City cases2. Sample for the present study was taken between the time of admission and positive results on the virus strain (AH1N1) through RT-PCR assay (2 days) before receiving any medication for the illness. At the time of admission thirty-six (90%) of study patients had increased lactase dehydrogenase (DHL) levels ranged from 511–1585 IU per liter. Twenty patients (50%) presented lymphocytopenia (low-normal lymphocytes account <1,000 per cubic millimeter). Three patients presented elevated creatinine levels (2.32–3.45 mg per deciliter). Fifteen cases (38%) presented lower than referenced hemoglobin values (Hb ≤ 12 grams per deciliter). Eight cases were off threshold than referenced values in hematocrit (36%–52%). All selected cases with unknown history of occupationally toxic-metals exposure. Table 2 presents characteristics of AH1N1 cases selected for the study.
Controls I and II
The start time of symptoms and admission/diagnosis in the hospital was between 5–7 and 3–5 days for Controls I and II, respectively. Controlled cases presented identical symptoms to those featured for the key cases, negative for the pandemic strain, all recovered from illness. They presented unknown history of occupationally toxic-metals exposure and went through blood tests as part of the research protocol approved by the National Council of Science and Technology of Mexico (CONACyT-Reference 126657-2009-02) during pandemic year. Sample for control groups was taken between the time of admission and negative results on the RT-PCR assay (2 days) before receiving any medication for the illness. Main characteristics of Controls I and II are summarized and compared to AH1N1 cases in Table 2. Details of both groups are described in Methods Section.
Critical care cases: survivals and non-survival AH1N1 patients
Ten of our AH1N1 cases received tracheal intubation; seven of these not survived. There were a total of 8 non-survivals AH1N1 patients documented in this research. All of them corresponded to samples taken in 2009 (1st October to 31st December). None of the non-survival patients received 2009 AH1N1 or seasonal influenza or pneumococcal vaccination before illness and hospitalization. All of these cases had obesity in some extent (5 cases: type I; 1 case: type II; 2 cases: morbid). Only three of them had a history of current smoking, suspended at the time of presenting symptoms. Mean age of non-survival patients (8/40) of the study was 45.33 years.
Multi-element determination in whole blood of AH1N1 cases and controls
28–31 metals and metalloids were accurately reproducible through evaluation of certified and referenced standards described in Methods Section. Results of the present study are focused on whole blood concentration levels of the following elements: lead, mercury, chromium, cadmium, arsenic and selenium, zinc, copper, calcium and sodium.
(a) Potentially-toxic metals profile for cases and controls analysed
Table 3 describes statistic parameters for concentration levels of lead, mercury, cadmium, arsenic and chromium in whole blood of cases and controls analysed. Table 4 presents statistical analysis results for lead and mercury heavy-metals. Details of statistics approaches applied to evaluate significant differences between cases and controls are depicted in Methods section.
Lead (Pb)
Blood lead levels ranged from 43.77 to 366.18 ppb for AH1N1 cases and from 2.04 to 112.7–191.6 ppb for controls. Mean blood lead levels for cases were greater than literature7 values (62.8 ppb) 75% of the time (30/40 cases) while for Controls I and II this was registered 27% (8/30 controls) and 22% (14/64 controls) of the time, respectively. Mean blood lead levels for AH1N1 patients were two and almost three times larger than Controls I and II, respectively while median values were almost twice (1.9) larger versus Controls I and more than 3 times (3.3) than Controls II. Two cases (of the 40 AH1N1) had blood lead levels greater than the threshold (≤250 ppb for adults) delimited by Mexican Norm8, while ten (25%) of the total cases had levels greater than recommended values by the Center for Diseases Control (≤150 ppb in young adults). Nonetheless, seven of these ten cases recovered from the illness and survived. Statistical analysis (Table 4) showed blood lead levels of AH1N1 patients versus Controls I and II met highly statistical significance while lead concentrations between subgroups of Controls (I versus II) did not.
Mercury (Hg)
Mean blood mercury levels for cases (AH1N1) and Controls (I and I) were slightly higher than threshold referenced7,9,10 values (mean 1.1 μg/dL). As shown in Table 4, statistical analysis between blood mercury levels of pneumonia –infected AH1N1 patients versus Controls II (ILI cases) met highly statistical significance while between Controls I (pneumonia cases) and controls II did not. Probable reasons for Hg blood levels found among population living in Mexico City during pandemic influenza might be the content of mercury in airborne particulate matter recently reported in literature11. Mercury belongs to one of the most harmful xenobiotic with severe implications (e.g., kidney impairment) although chronic low-dose exposure remains still not well understood9.
Chromium (Cr)
Chromium blood levels for AH1N1 patients ranged from 0.278 to 8.94 μg/dL; mean of 4.604 ± 1.94 SD μg/dL, greater than literature12 reported values. Cr (as well as Cd and As) determinations in samples of control group (I) were not achieved for this pandemic study. In spite of this, samples of cases and controls II analysed showed mean and median blood levels of AH1N1 patients were substantially higher, almost four (3.9) and five (4.8) times, than concentration levels found for this elements in the control group –II. Statistical analysis is not performed for Chromium as more than 50% of the values are within the lower detection limit (LDL) of the analytical technique, as detailed in Table 3.
Cadmium (Cd)
Blood cadmium levels in AH1N1 patients ranged from 1 to 21.79 ppb. Overall mean 10.7 ± 2.63 SD ppb, remarkably larger than referenced7,13 values (0.4–4.0 ppb). Cadmium high levels have been related to an increased risk to organ failure. Exposure to Cd can also result in damage to a variety of organ systems14,15. Cadmium, at levels greater than referenced values, represents a serious threat particularly to the vascular endothelium, the immune system, the nervous system and even the local chemical/metabolic environment of individual organs16,17,18. Nevertheless, only two AH1N1 cases having highest Cd concentrations did not recover from illness. As seen in Table 3, more than 50% of the Cd concentrations of both cases and controls (II) were detected within the LDL resulting in an important limitation to evaluate statistical significance between subgroups.
Arsenic (As)
Blood arsenic levels for AH1N1 patients ranged from 1 to 44 ppb; mean of 8.62 ± 12.27 SD ppb, remarkably greater than literature7 values (range: 2.6–17.8 ppb; mean: 5.0 ppb). Indeed, seven of our eleven AH1N1 cases having the highest As concentrations (range: 11–36 ppb) did not recover from illness. Nevertheless, as seen in Table 4, more than 50% of the As concentrations of both cases and controls (II) were detected within the LDL resulting in an important limitation to evaluate statistical significance between subgroups.
(b) Essential elements profile for cases and controls analysed
Table 5 describes statistic parameters for concentration levels of selenium, zinc, calcium, sodium and cooper in whole blood of analyses cases and controls I and II. Table 6 presents statistical analysis results for selenium (Mann-Whitney/Bonferroni correction) and the rest of essential elements (Mann-Whitney U Tests). Statistics analysis approaches are detailed in Methods section.
Selenium (Se)
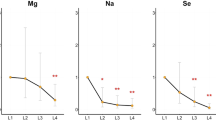
Mean blood Se levels for AH1N1 was 10.62 μg/dL, versus controls I and II concentrations of 13.67 and 15.46 μg/dL, respectively; within reported7,13,19 values for young population (mean range: 11.0 to 15.0 μg/dL). Overall blood selenium values in key AH1N1 cases tended to decrease versus controls I and II. This is in agreement with results showed in Table 6 where blood selenium levels of AH1N1 patients were statistically significantly lower when compared to both Controls I and II. It is also noted that selenium blood levels also reached statistical significance for Controls I (pneumonia) when compared to controls II (ILI).
Survival and non-survival AH1N1 cases
Cases with selenium concentrations greater than 12.5 μg/dL-level, suggested as the optimal cut-off for glutathione peroxidase activity20 recovered from illness in fewer days (5–7 days) than the average time (10–12 days for pneumonia; 15–21 days for pneumonia –AH1N1 infected ones) registered for cases analysed during pandemic year at INER. Seven (7/8) non-survival cases registered selenium levels lower than the optimal cut-off abovementioned. Four of critical patients presenting severe renal failure reached the lowest Se values: 8.23, 7.81, 7.07, 6.21 μg/dL accordingly with progression (three different samples collected during 15–21 days of hospitalized period) and severity of the illness and finally did not recover from it. Levels of selenium of cases abovementioned were similar to that reported20 for pregnant women who tend to decrease when increasing time of gestation, 1st trimester: 7.55–8.5 μg/dL; 2nd: 7.2–8.1 μg/dL; 3rd: 6.99–7.75 μg/dL21. Pregnancy has been set as a determinant risk factor (increasing fatal evolution with time of gestation) for reported cases3 during pandemic influenza although none of the cases under discussion in our study corresponded to this condition. The concentration of selenium in whole blood varies widely between geographic areas depending on its content in soil, dietary Se intake, retention and interaction with other minerals in the body organism22. Despite the fact that Mexico City is not particularly considered as low-selenium area, a supplement of this essential metal might be considered on the recovery of pneumonia patients with particular emphasis on those infected by AH1N1 strain, as analysed in this research.
Zinc (Zn)
Mean blood zinc levels for cases corresponded to 669.48 ± 260.01 SD μg/dL, very similar to those obtained for controls II (662.469 ± 271.8 SD μg/dL). Zinc (as well as calcium, sodium and copper) determinations in samples of control group (I) were not achieved for this pandemic study. As shown in Table 5, mean and median values of both cases and controls are in the inferior value limit of literature13,10 (range: 600–990 μg/dL) and significantly lower than values reported recently for young Mexican population23 (930–1340 μg/dL). No statistically significant differences were observed when compared Zn blood levels of AH1N1 cases versus controls, as presented in Table 6. Nevertheless, 62% of lowest Zn values (282–586 μg/dL) registered for key cases did show a correlation (R = 0.978) with corresponding higher lead levels (≥15 μg/dL). Adequate Zn concentrations have been suggested to be effective as a preventive way for pneumonia infection24 nevertheless whether nutritional deficiencies affects bronchial epithelial cells and modify susceptibility of viral influenza remains not well understood.
Zinc concentrations in blood are a key feature in the analysis of Zn to Cu ratios of cases and controls presented below.
Survival and non-survival patients
There were no substantial differences in blood Zn levels between survivals and non-survivals for AH1N1cases analysed here.
Calcium (Ca)
Blood calcium levels for cases ranged from 1.8 to 4.82 mg/dL; mean value of 3.98 mg/dL. For controls II ranged concentrations ranged from 1.77–4.94 mg/dL; mean value of 3.90 mg/dL, both below literature values (5.75 to 10 mg/dL). Calcium concentrations were not achieved for samples of controls-I as aforementioned. No statistically significant differences were observed when compared calcium blood levels of cases versus controls II, as shown in Table 6. Nevertheless, lesser calcium values (30% lower than mean values) corresponded 60% of the time with higher lead values (≥14–15 μg/dL) for AH1N1 cases under study.
Survival and non-survival patients
There were no substantial differences in blood Ca levels between survivals and non-survivals for AH1N1cases analysed here.
Sodium (Na)
Blood sodium levels for key cases ranged from 188.04 to 316.04 mg/dL; mean value 271.95 mg/dL. Blood sodium levels for controls –II ranged from 260–317 mg/dL; mean value of 286.72 mg/dL; slightly below literature values (300–320 mg/dL). Sodium concentrations were not achieved for samples of controls-I as aforementioned. No statistically significant differences were observed when compared sodium blood levels of cases versus controls II, as shown in Table 6.
Survival and non-survival patients
There were no substantial differences in blood Na levels between survivals and non-survivals for AH1N1cases analysed here.
Copper (Cu)
Blood copper levels for AH1N1 patients ranged from 64.34 to 163.74 μg/dL and 102 to 208 μg/dL for controls II. Mean values of 111.55 and 127 μg/dL for cases and controls -II, respectively, both within literature (80–140 μg/dL). Copper concentrations were not achieved for samples of controls -I as aforementioned. Significance was achieved for Cu concentrations levels between AH1N1 cases and controls –II as shown in Table 6.
A metabolic imbalance is observed when deficiency of the essential element resulted in higher ratios of zinc to copper for AH1N1 influenza cases under study (6.07) versus their controls (5.52). It is also noted that Cu blood levels in AH1N1 patients tended to decrease (and ratios of zinc to copper tended to increase) accordingly with obesity, in agreement with previous studies25. For AH1N1 cases analysed in this research this was particularly observed for category III (morbid).
Survival and non-survival patients
Differences in blood copper levels between survivals and non-survivals AH1N1 cases were observed when a pre-condition (particularly, obesity III) occurred. Number of cases to evaluate significance between copper levels was a limitation to perform statistical analysis between these two subgroups.
Discussion
We have showed that whole blood (WB) profile of potentially-toxic heavy metals in pneumonia -AH1N1 infected patients was statistically significant (Pb, Hg) and remarkably (Cd, Cr, As) different when compared with WB levels of pneumonia and ILI controls, both negative for the pandemic strain. Higher WB lead concentrations (≥14–15 μg/dL) for cases under study were related (in 38% of the cases) to a lower than referenced values of hemoglobin; lesser concentrations of Ca2+ and Zn2+ (60–62% of the time) were registered for aforementioned concentrations as well. Substitution of essential elements by lead has been widely mentioned in literature though underlying mechanisms have not been fully elucidated26. Spite the fact that key cases and controls were selected accordingly to specific guidelines of the research protocol regarding “no exposure to a specific environmental source of toxicity related to the metals and metalloids considered in the study”, concentration levels of some potentially-toxic metals (e.g. Pb) found in this study might be, in part, caused by an unknown source determined by environmental conditions of Mexico City inhabitant's resident such as legacy of past domestic uses (leaded paints or ceramics) as well as environmental exposure to dust and soil remissions containing heavy-metals27. Environmental exposure to lead through airborne particulate matter might be considered with reserves as leaded gasoline was phase-out from Mexico by federal and local stricter regulations on this matter since 199728. On the other hand, potentially-toxic blood levels of at least three of the five heavy-metals studied (Pb, Cd, Cr) were remarkably higher in AH1N1 –infected patients segregated by smoking factor. Indeed lead and cadmium high levels have been related unequivocally to the condition of smoking15 and increased organ failure14,15. Nevertheless, statistical analysis was not conclusive on this matter (inhomogeneity of different subgroups), particularly in the context of severity of the illness (survival or non-survival patients), at least for cases under study. Along with significant concentrations of potentially-toxic elements, deficiency of some essential metals (Se, Cu) characterized AH1N1 viral-infection cases in Mexico City. WB levels of selenium in AH1N1 patients remained statistically significantly (p<0.001) lower (mean: 10.42 μg/dL) than controls I and II (means 13.67; 15.19 μg/dL, respectively). It is noted highly statistically significant decrement of selenium from controls II (influenza like illness) through controls I (pneumonia) and finally in pneumonia -AH1N1 infected patients, the latter registering the lowest values of the essential element. Selenium values for critical (ICU) patients who survived from those who did not, capture our attention. Results showed that all AH1N1 patients having blood Se levels greater than the recommended level for an optimal cut-off to activate glutathione peroxidase (12.5 μg/dL) recovered from the illness in a shorter time and survived. Nevertheless the effect of selenium status on responses to viral infection is not fully understood, selenium depletion has been shown in a cell culture model to affect influenza virus-induced cytokine production in bronchial epithelial cells29. With regards to renal failure (non-survival cases), it should be noted that this condition further decreases Se levels and glutathione peroxidases in blood30. Based on clinical-lab features of ICU patients who survived from those who did not, evaluation of key nutrients, such as selenium, is currently under evaluation in the recovery of pneumonia -viral -infected patients at the National Institute for Respiratory Diseases. Finally, increased Zn/Cu ratios were observed in AH1N1 patients versus controls not infected with the pandemic strain. Furthermore, Zn/Cu ratios were remarkably high for AH1N1 cases where obesity (category II-III) was a pre-existing condition. This enforces results discussed by the WHO consultation on clinical aspects of pandemic influenza3.
Methods
Study participants
AH1N1 patients
The pandemic influenza study was conducted at INER as part of a national public health investigation during the collection of AH1N1 samples and review of clinical data (2nd semester 2009-1st semester of 2010). Selection of the key AH1N1 cases was done accordingly to standards and specifications of the research protocol approved by the National Council of Science and Technology in Mexico (CONACyT-Reference 126657) in 2009. The pandemic influenza study was also approved by the Committee of Science and Bioethical of the National Institute of Respiratory Diseases in the same year. All patients selected had pneumonia and positive result on reverse-transcriptase-polymerase-chain-reaction (RT-PCR) testing for influenza A (H1N1) according to guidelines from the U.S. Center for Control Diseases and Prevention (CDC) published during pandemic influenza31. All participants received Institutional informed written consent also approved by Institutional Committee of Bioethics and Science at INER, before the sample collection and laboratory tests.
Control subjects
Two groups of controls were selected for purposes of our study. Controls -I corresponded to thirty (30) subjects admitted and diagnosed with pneumonia but negative for the pandemic strain. Controls –II corresponded to 64 subjects directly exposed to the AH1N1 virus in Mexico City diagnosed as influenza like illness (ILI) and also negative for the pandemic strain. Controls were persons in contact with an AH1N1 patient and were selected at both National Institutes for Respiratory Diseases (INER) and Medical Sciences and Nutrition (INCMNSZ) accordingly to guidelines detailed in the research protocol aforementioned. Sample collection of controls initiated during first outbreak and extended throughout WHO pandemic year. They were selected according to the age range of influenza patients (20–55 years), having unknown history of occupational toxic exposure and with any influenza (AH1N1 or seasonal) or pneumococcal vaccines previously to blood sample was taken. Criteria for selection of the control during pandemic period was being one of the closest AH1N1 patient's relatives (not by blood), exposed to the virus through direct contact with him/her for at least three (3) days before patient's hospitalization. All participants received medical consultation, blood (biometry and toxicology) tests accordingly to the CONACyT research protocol and were selected having unknown history of pre-existing health conditions. Controlled cases received Institutional informed written consent also approved by the Committee of Bioethics and Science at INER, before the sample collection and laboratory tests. Both INER and INCMNSZ are National Institutes and public hospitals that serve a low-moderate income population mainly from Mexico City and its Metropolitan Area. For inclusionary and exclusionary criteria of selection as a “control” for pandemic influenza research protocol, reader is referred to Table 1.
Clinical-lab data
Clinical-medical charts of the 40 patients hospitalized for pneumonia at INER were reviewed. Real Time-Polymerase Chain Reaction (RT-PPCR) of all patients was positive for AH1N1 at admission though nasopharyngeal-swab sample or after tracheal intubation for Intensive Care Unit (ICU) cases. All procedures for RT-PCR assay were followed according guidelines provided by the U.S. CDC in 2009. Age, gender, history of smoking, diabetes mellitus or other pathologies, body mass index (BMI) as well as laboratory clinical levels of serum lactase dehydrogenase (DHL), leukocyte counts, creatinine, hemoglobin and hematocrit levels at admission were compiled in the clinical-lab database for the study.
Blood sample collection
Trained medical and research staff performed all sample collection at the National Institutes (INER and INCMNSZ) in Mexico City, Mexico. Original plus replicate whole-blood samples of 3–5 ml were collected in metal-free tubes for multi-elemental trace element analyses. All whole-blood collection and processing materials were acid cleaned, according to Mexican Norms8. All reagents were ultra-high purity or ultrapure analytical grade. Selected samples for the protocol were frozen at -20°C at the INCMNSZ until chemical analyses, according to sample collection guidelines for trace elements in blood32.
Trace elements quantification
28–31 metal and metalloid determination were quantified from 60 acid-digested whole blood samples (AH1N1 patients' samples plus blanks and calibration standards) through an Inductively-Coupled Plasma Mass Spectrometer (ICP-MS; Agilent Technologies, U.S.) and an Atomic Absorption Spectrometer (AAS; Perkin Elmer, U.S.) at Laboratory of Instituto de Ciencias del Mar y Limnología from the National Autonomous University of Mexico (UNAM). Elements determined through ICP-MS were Li, Mg, Al, K, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Ge, As, Se, Sr, Y, Mo, Ag, In, Sn, Sb, Ba, Tl, Pb, Hg and Bi. Lead and zinc quantifications were double-verified by AAS– graphite furnace; technique that was also applied for cadmium determination. Mercury was determined by AAS- hydride generation. Finally, calcium and sodium measurements in whole blood were performed by AAS-flame. The three variants (flame, hydride generation and graphite furnace) of the AAS technique are literature recommended techniques for quantification of metals and metalloids of interest. The CONACyT research protocol was also subject to external quality control on the elemental analysis to verify ICP-MS and AAS determinations of the core (blood) samples of AH1N1 patients through an Inductively Coupled Plasma- Collision Cell- Mass Spectrometer (ICP-CC-MS) at the Instituto de Geología from the Autonomous University of San Luis Potosí (UASLP), San Luis Potosí, Mexico. Details of the analytical technique are depicted elsewhere33. As part of quality control program and the inter-comparison of internal and external laboratories we applied same certified referenced materials from the International Atomic Energy Agency (IAEA-A-13 freeze dried animal blood) and High Purity (CRM-Bovine Liver) to evaluate accuracy and reproducibility of the analytical methods aforementioned. ICP-MS determinations reported here had an adequate linearity, accuracy and precision during the quantification study. Briefly, the method yields a measurement precision of ≤0.5%RSD for lead (reference potentially-toxic element) concentrations of <0.003 μg/ml. Trace-elements from control samples were also double-verified by ICP-CC-MS following the same ICP-MS methodology and certified standards. The analytical detection limit determined was 0.1 μg/dL for ICP-CC-MS and 1–2 μg/dL for AAS. Metal and metalloid concentrations are reported in micrograms per deciliter (μg/dL) or micrograms per liter (ppb), unless otherwise indicated.
Statistical Analysis
Non-parametric analysis was applied to evaluate significant differences on concentrations of potentially-toxic elements and essential elements in whole blood of AH1N1 patients versus their controls. This variant was chosen as at least one assumption, e.g. independence and normality or homogeneity of variances was not adequate to perform one-way ANOVA analysis. Kruskal-Wallis test was applied to evaluate significant differences concentrations of potentially-toxic elements lead and mercury and essential element selenium in whole blood of AH1N1 patients versus their controls I and II. Mann-Whitney Post Hoc test was performed for pairwise multiple comparisons with a Bonferroni correction to achieve that no more than a five percent of chance of making a type-1 error for any of the hypotheses being tested was present. For the rest of essential elements: zinc, copper, calcium and sodium Mann-Whitney U test was applied to evaluate if significant differences were observed between concentration levels in whole blood of AH1N1 patients versus their respective controls (II). Data reported in the present study is expressed as mean ± SD (standard deviation), median and IQR (Inter-quartile range). Significance was set as p<0.05; highly statistical significance was considered as p<0.001.
References
World Health Organization: World now at the start of 2009 influenza pandemic (2009). http://www.who.int/meadicetre/news/statements2009/h1n1_pandemic_phase6_20090611/en/index.html. Accessed on September 11, 2010.
Pérez-Padilla, R. et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. New Engl. J. Med. 10.1056/NEJMoa0904252 (2009).
Echevarría-Zuno, S. et al. Infection and death from influenza A H1N1 virus in Mexico: a retrospective analysis. Lancet 374, 2072–2079 (2009).
World Health Organization: Clinical aspects of pandemic 2009 Influenza A (H1N1) virus infection. Writing Committee of the WHO Consultation on Clinical Aspects of pandemic (H1N1) 2009 Influenza. New Engl. J. Med. 362, 1708–19 (2010).
Chowell, G. et al. Characterizing the epidemiology of the 2009 influenza A/H1N1 pandemic in Mexico. PLoS Med. 8 (5), e10000436 (2011).
World Health Organization: In focus. H1N1 now in post-pandemic period (August 10, 2010). http://www.who.int/csr/disease/swineflu/en/index.html. Accessed on December 31st, 2011.
Goullé, J. P. et al. Metal and metalloid multi-elementary ICP-MS validation in whole blood, plasma, urine and hair. Reference values. Forensic Sci. Intl. 153, 39–44 (2005).
NOM-199-SSA1-2000. Norma Oficial Mexicana Salud Ambiental. Niveles de Plomo en sangre y acciones como criterio para proteger la salud de la población expuesta no ocupacionalmente. Secretaría de Salud, México. (2002).
Gundacker, C. et al. Whole blood mercury and selenium concentrations in a selected Austrian population: Does gender matter? Sci.Total Environ. 372, 76–86 (2006).
Bárány, E. et al. Trace elements in whole blood and serum from Swedish adolescents. Sci. Total Environ. 286, 129–141 (2002).
Rutter, A. P. et al. In situ measurements of speciated atmospheric mercury and identification of source regions in the Mexico City Metropolitan Area. Atmos. Chem. Phys. 9, 207–220 (2009).
Iyengar, V. & Wolttlez, J. Trace elements in human clinical specimens. Evaluation of literature to identify Reference values. Clin. Chem. 34 (3), 474–481 (1998).
Moreno, M. A., Marín, C., Vinagre, F. & Ostapczuk, P. Trace elements levels in whole blood samples from Badajoz, Spain. Sci. Total Environ. 229, 209–215 (1999).
Navas-Acien, A. et al. Lead, cadmium and smoking and increased risk of peripheral arterial disease. Circulation 109, 3196–3201 (2004).
Jarup, L., Berglund, M., Elinder, C. G., Nordberg, G. & Vahter, M. Health effects of cadmium exposure- A review of the literature and a risk estimate. Scand. J. Work Environ. Health 21, 1–51 (1998).
Triggle, C. R. et al. The endothelium in health and disease- A target for therapeutic intervention. J. Smooth Muscle Res. 39, 249–267 (2003).
Villar, I. C., Francis, S., Webb, A., Hobbs, A. J. & Ahluwalia, A. Novel aspects of endothelium-dependent regulation of vascular tone. Kidney Int. 70, 840–853 (2006).
Prozialeck, W. et al. The vascular system as a target of metal toxicity. Toxicol. Sci. 102, 207–218 (2008).
Sabé, R., Rubio, R. & García-Beltrán, M. . Determination of selenium in human blood specimens by electrothermal atomic absorption. Review. Anal. Chim. Acta 419, 121–135 (2000).
Thompson, C. D. Assessment of requirements for selenium and adequacy of selenium status: a review. Eur. J. Clin. Nutri. 58, 391–402 (2004).
Ferrer, E. et al. Whole blood selenium content in pregnant women. Sci. Total Environ. 227, 139–143 (1999).
Wasowicz, W., Gromadzinska, J., Rydzynski, K. & Tomczak, J. Selenium status for low-selenium area residents: Polish experience. Toxicol. Letters 137, 95–101 (2003).
Martínez, T. et al. Determination of trace elements in blood samples by TXRF analysis. J. Radioanal. Nuclear Chem. 259, 511–514 (2004).
Black, R. Zinc deficiency, infectious diseases and mortality in the developing world. 11th Meeting of the international organization “trace elements in man and animals. (TEMA)”, BerkeleyCalifornia June 2–6 (2002).
Sánchez, C., López-Jurado, M., Aranda, P. & Llopis, J. Plasma levels of copper, manganese and selenium in an adult population in Southern Spain. Influence of age, obesity and lifestyle factors. Sci. Total Environ. 408, 1014–1020 (2010).
Gordon, J. N., Taylor, A. & Benett, P. N. Lead poisoning: cases studies. Br. J. Clin. Pharmacol. 53 (5), 451–458 (2002).
Martínez, T., Lartigue, J., Avila-Perez, P., Zarazúa, G., Cabrera, L., Tejeda, S. & Ramírez, A. Determination of lead in blood by TXRF and its correlation to environmental lead. Nuclear Instrum. Methods Phys. Research B. 213, 584–589 (2004).
Flores, J. & Albert, L. Environmental lead in Mexico. Rev. Environ. Contam. Toxicol. 181, 37–109 (2004).
Fairweather-Tait, S. et al. Selenium and human health and disease. Antioxidants and Redox Signaling 14 (7), 1337–1383 (2011).
Zachara, B., Salak, A., Koterska, D., Manitius, J. & Wasowicz, W. Selenium and glutathione peroxidases in blood of patients with different stages of chronic renal failure. J. Trace Elem. Med. Biol. 17,291–299 (2004).
CDC Protocol of real-time RTPCR for influenza A (H1N1). Geneva: World Health Organization, April 2009 (accessed on December 31st 2011, at http://www.who.int/csr/resources/publications/swineflu/CDCRealtimeRTPCR_SwineH1Assay-2009_20090430.pdf ).
Cornellis, R. et al. Sample collection guidelines for trace elements in blood and urine. Pure Appl. Chem. 67, 1575–1608 (1995).
Guzmán-Morales, J. et al. Assessment of atmospheric metal in the urban area of Mexico City using ficus benjamina as biomonitor. Bull. Environ. Contam. Toxicol. 86, 495–500 (2011).
Acknowledgements
We extend our gratitude to patients and volunteers that participated in the study. Research was funded by the National Council of Science and Technology (CONACyT-Salud 126657). Residents and graduate students of INCMNSZ & INER: J. Flood, P. Ortega, E. Olaya and R. Zenteno-B are acknowledged for invaluable help. M. Moya recognizes Dr García-Colín Scherer who enlightened the paper in the understanding of biochemical non-equilibrium features of key cases.
Author information
Authors and Affiliations
Contributions
M.M. conceived and directed the toxicology AH1N1 study and wrote the paper. E.G.B. evaluated clinical aspects of critical and non-critical patients. A.V.-G. evaluated medical and laboratory blood tests for the collection and selection of blood samples for the research. G.T. performed statistical analysis. F.V.G. designed the protocol for the pre-treatment of viral-infected samples at UNAM. F.V.G. & M.E.G.-A. quantified toxicology and essential multi-element profiles in blood samples of AH1N1 and controls. M.C. & A.H. (Epidemiology) helped with controlled cases.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Moya, M., Bautista, E., Velázquez-González, A. et al. Potentially-toxic and essential elements profile of AH1N1 patients in Mexico City. Sci Rep 3, 1284 (2013). https://doi.org/10.1038/srep01284
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep01284
This article is cited by
-
Selenium in the Prevention of SARS-CoV-2 and Other Viruses
Biological Trace Element Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.