Abstract
Group II metabotropic glutamate receptors (mGluR2/3) have emerged as important targets for the treatment of schizophrenia. Since hypofunction of N-methyl-D-aspartate receptors (NMDARs) has also been implicated in the etiology of schizophrenia, we examined whether postsynaptic mGluR2/3 regulate NMDAR function. Activation of mGluR2/3 significantly decreased the ratio of AMPA-to-NMDA excitatory postsynaptic currents at Schaffer Collateral-CA1 synapses and enhanced the peak of NMDA-evoked currents in acutely isolated CA1 neurons. The mGluR2/3-mediated potentiation of NMDAR currents was selective for GluN2A-containing NMDARs and was mediated by the Src family kinase Src. Activation of mGluR2/3 inhibited the adenylyl cyclase-cAMP-PKA pathway and thereby activated Src by inhibiting its regulatory C-terminal Src kinase (Csk). We suggest a novel model of regulation of NMDARs by Gi/o-coupled receptors whereby inhibition of the cAMP-PKA pathway via mGluR2/3 activates Src kinase and potentiates GluN2A-containing NMDAR currents. This represents a potentially novel mechanism to correct the hypoglutamatergic state found in schizophrenia.
Similar content being viewed by others
Introduction
Glutamate is the primary excitatory neurotransmitter in the brain and binds both ligand-gated ion channels such as the N-methyl-D-aspartate receptor (NMDAR) and G protein-coupled metabotropic receptors (mGluRs). Hypofunction of the NMDAR (a hypoglutamatergic state) represents a major hypothetical mechanism explaining the etiology of schizophrenia1,2,3. This hypothesis is based on initial observations that non-competitive NMDAR antagonists induce a transient psychosis, disrupt affect and impair cognitive function in healthy humans and can exacerbate preexisting symptoms in patients with schizophrenia4,5. Furthermore, NMDAR-mediated signaling and GluN2A tyrosine phosphorylation is significantly reduced in postmortem brains from schizophrenia subjects3. The NMDAR pathway is associated with several candidate genes for schizophrenia, including neuregulin-1, dysbindin, disrupted-in-schizophrenia 1 and metabotropic glutamate receptor 3 (mGluR3)6; thus, NMDAR-mediated signaling is proposed to act as a point of convergence for various candidate pathways in this disorder.
Accumulating evidence suggests that the group II family of mGluRs (mGlu2 and mGlu3; mGluR2/3) may represent an important target in the treatment of schizophrenia7,8. In NMDAR hypofunction models of psychosis, agonists of mGluR2/3 reduce phencyclidine- and dizocilpine-induced locomotor behaviours9,10,11. A recent phase II clinical trial showed that LY 214 0023, an oral prodrug of the selective mGluR2/3 agonist LY 404039, was effective in treating both positive and negative symptoms of schizophrenia after only 4weeks of treatment8. Furthermore, group II mGluRs are highly expressed in regions of the brain associated with schizophrenia such as the prefrontal cortex and hippocampus12,13. Although there is ample evidence that mGluR2/3 agonists are effective in schizophrenia, the exact mechanisms for their antipsychotic effects are unclear.
Group II mGluRs are predominantly expressed on presynaptic terminals where they inhibit release of glutamate and GABA14. Activation of postsynaptic mGluR2/3 negatively modulates neuronal excitability and plasticity15,16. Given that mGluR2/3 mainly act presynaptically to inhibit glutamate release, it seems counterintuitive that activation of these receptors would ameliorate the hypoglutamatergic state found in schizophrenia. Therefore we examined the effects of mGluR2/3 activation on NMDA-evoked currents in identified CA1 pyramidal neurons of the hippocampus, a brain region implicated in the pathophysiology of schizophrenia17,18.
Results
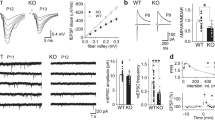
We initially examined the effects of mGluR2/3 activation on NMDAR-mediated field EPSPs (fEPSPNMDA) in the CA1 region. Application of the selective mGluR2/3 agonist LY 379268 (30nM) did not affect the amplitude of fEPSPNMDA (Fig. 1a); however, there was an increase in the variance of the fEPSPNMDA response, suggesting that postsynaptic NMDAR function may be enhanced in spite of a reduction in presynaptic release of glutamate. To determine whether mGluR2/3 activation enhances postsynaptic NMDAR function, we examined the AMPA/NMDA ratio of excitatory postsynaptic currents (EPSCs) at Schaffer Collateral-CA1 synapses. Application of LY 379268 (30nM) resulted in a significant decrease of AMPA/NMDA EPSC ratio (p<0.05, Fig. 1b), suggesting an enhancement of postsynaptic NMDAR function. To more directly examine the effects of mGluR2/3 activation on NMDARs, we examined the actions of LY 379268 on acutely isolated CA1 pyramidal neurons.
Activation of group II mGluRs enhances postsynaptic NMDAR function.
A) Bath application of LY 379268 (30 nM) to hippocampal slices does not change the amplitude of NMDAR-mediated fEPSPs relative to baseline (N = 6). Below, quantification of NMDA-fEPSP change before and after application of LY 379268. B) Stimulation of mGluR2/3 with LY 379268 (30nM) significantly decreased the AMPA-NMDA EPSC ratio (N = 11) relative to baseline (p<0.05). Below, example traces showing EPSCs at +40mV and 70mV from control and LY 379268-treated slices, respectively; AMPA EPSC amplitude was measured at 70mV and NMDA EPSC amplitude was measured at +40mV. * Indicates p<0.05, Student's t-test.
We previously demonstrated that Gq- and Gs-coupled receptors potentiate NMDAR currents in isolated CA1 neurons via activation Src kinase and PKA, respectively19,20,21,22,23. Given that mGluR2/3 couple to Gi/o and inhibit adenylyl cyclase activity14, we anticipated that these receptors would inhibit NMDAR-mediated currents. To examine the direct effects of group II mGluRs on NMDARs, we determined the actions of LY 379268 on acutely isolated CA1 pyramidal neurons. These isolated neurons have a population of both extrasynaptic and synaptic NMDARs. Surprisingly, application of LY 379268 (10nM) to acutely isolated CA1 neurons potentiated NMDA-evoked currents, with a significant potentiation occurring after washout of LY 379268 (Fig. 2). The concentration of LY 379268 employed was chosen based on the 50% percent inhibitory concentration (IC50) value of LY 379268 to displace 3HLY341495, a group II selective antagonist radioligand, from native rat brain homogenates and recombinant human mGlu2 and mGlu3 receptor subtypes24. The LY 379268-induced enhancement of NMDAR-mediated currents was blocked by co-application of the selective mGluR2/3 antagonist LY 341 495 (10nM) (Fig. 3). Conversely, co-application of the selective group I mGluR antagonist, MPEP hydrochloride, did not prevent the enhancement of NMDAR currents by LY 379268 (Fig. 3). These results confirm that the LY 379268-mediated potentiation of NMDAR currents was indeed mediated by mGlu2/3 receptors. Given that PKA promotes Ca2+ permeation through NMDARs and increases the amplitude of NMDA-evoked currents25, it seems counterintuitive that inhibition of the PKA pathway via group II mGluRs would mediate the enhancement of NMDAR currents. Instead, we hypothesized that stimulation of group II mGluRs results in a promiscuous activation of Gq with a subsequent activation of PKC and/or Src leading to an enhancement of NMDAR currents19,20,22,23. To test this hypothesis, we employed the Src(4058) peptide which mimics the unique domain of Src and prevents its interaction with the NADH dehydrogenase subunit 2 domain in the NMDAR complex26. Thus, Src(4058) acts as an interfering peptide to prevent the regulation of NMDARs by endogenous Src26. Including Src(4058) in the patch pipette prevented the enhancement of NMDAR currents by LY 379268 (Fig. 4a). The LY 379268-induced enhancement of NMDAR currents was not, however, prevented by application of the PKC inhibitor, bisindolylmaleimide I (0.5 M, data not shown) suggesting that Src might be activated by an alternative mechanism. Using an approach similar to Gingrich et al.26, we synthesized the Fyn interfering peptide, Fyn (3957) that corresponds to a region of the unique domain of Fyn. We previously showed that Fyn(3957) selectively blocks the potentiation of NMDAR currents by recombinant Fyn kinase but not by recombinant Src kinase23. Application of Fyn(3957) inside the patch pipette failed to prevent the enhancement of NMDAR currents by LY 379268 (Fig. 4a) indicating that LY 379268 enhances Src but not Fyn activity to regulate NMDARs. To confirm these electrophysiological findings, we determined the relative activation of Src versus Fyn in hippocampal slices treated with or without LY 379268 (30nM). The mGluR2/3 agonist enhanced phosphorylation of Src at Y416 (Fig. 4b), a site whose phosphorylation is required for activation this kinase27. The LY 379268-mediated increase in Src Y416 phosphorylation was prevented by co-application of the mGluR2/3 antagonist LY 341 495 (Fig. 4b). In contrast, LY 379268 failed to enhance the tyrosine phosphorylation of the analogous activation site of Fyn kinase, Y420 (Fig. 4b)28. These findings illustrate that Src and not Fyn, regulates the mGluR2/3-mediated modulation of NMDAR currents in dissociated CA1 neurons.
Application of LY 379268 to acutely isolated CA1 neurons potentiates NMDAR currents.
Application of LY 379268 (10nM) to acutely isolated CA1 neurons (N = 18) resulted in an increase of NMDA-evoked peak currents that began after washout of the agonist and persisted for the rest of recording. NMDA-evoked currents in control cells (N = 10) remained stable throughout the time period of the recording. Cells treated with 10nM LY 379268 had significantly larger NMDA-evoked peak currents (control, 97.4 10%, N = 10; 10nM LY 379268, 120.8 14.6%, N = 18; p<0.001, unpaired t-test; data obtained at 20min. of recording). The shaded region indicates the period of LY 379268 application. Right, sample traces of NMDA-evoked currents for control and LY 379268-treated cells. Traces represent points immediately before LY379268 application (t = 5min.) and 10min. after the end of LY 379268 application (t = 2min.).
The LY 379268-mediated increase of NMDA-evoked currents is mediated by group II metabotropic glutamate receptors.
Upregulation of INMDA by LY 379268 (N = 11) was blocked by co-application of the selective mGluR2/3 antagonist LY 341495 (N = 9) (LY plus LY 341495, 99 1%, n = 7; LY alone, 131 2%, n = 10). The mGluR2/3 antagonist on its own did not change the amplitude of NMDAR-evoked currents (N = 7). Application of the group I mGluR antagonist MPEP hydrochloride failed to prevent the LY 379268-mediated potentiation of NMDAR currents (N = 9) (LY plus MPEP, 143 2%, n = 8; LY alone, 131 2%, n = 10). Antagonists (LY 341495, 10nM; MPEP hydrochloride, 10 M) were applied in all extracellular solutions for the duration of the experiment. The shaded region indicates the period of LY 379268 application.
mGluR2/3 activation recruits Src kinase to potentiate NMDAR currents in acutely isolated CA1 cells.
A) Quantification of normalized INMDA recorded from hippocampal CA1 neurons treated with LY 379268. mGluR2/3-mediated potentiation of INMDA (N = 11) is prevented by intracellular application of Src(4058) (N = 10) but not Fyn(3957) (N = 11) (LY plus Src(4058), 103 2%, n = 9; LY plus Fyn(3957), 125 2%, n = 10; LY alone, 135 2%). LY 379268 (10nM) was co-applied with NMDA/glycine solutions using the multi-barreled perfusion system. Fyn(3957), 25ng/ml and Src(4058), 25ng/ml, were included inside the patch pipette. Peak currents were averaged for 5min prior to application of LY 379268 and were compared with currents averaged for values between 20 and 25minutes. *Indicates P<0.01, one-way ANOVA. B) LY 379268 (30nM) treatment increases the phosphorylation of Src(pSrcY416) but not Fyn (pFynY420). The enhanced Src phosphorylation was prevented by pre-treatment with the mGluR2/3 antagonist LY 341495 (30nM). Below, summary of immunoblot analysis shows the averaged relative density of pSrcY416 and pFynY420 for each condition (n = 4). *Indicates P<0.05, Student's t-test.
We previously reported that several GPCRs acting via Gq activate Src kinase to phosphorylate the GluN2A subunit of this receptor22,23. In this regard, we employed applications of zinc (300 M) to selectively inhibit responses to heteromeric NMDARs containing two GluN2A subunits23,29. The enhancement of NMDAR currents by LY 379268 was absent when GluN2A subunits were blocked with Zn2+ (Fig. 5a). In contrast, the mGluR2/3-mediated modulation of NMDARs was still observed in the presence of the selective GluN2B antagonist, Ro 256981 (500nM) (Fig. 5a). We also examined the potential phosphorylation of GluN2A and GluN2B subunits and found that applications of LY 379268 enhanced tyrosine phosphorylation of GluN2A but not GluN2B subunits (Fig. 5b). Thus, activation of mGluR2/3 selectively potentiates GluN2A-containing NMDAR currents.
Stimulation of group II metabotropic glutamate receptors potentiates GluN2A-containing NMDAR currents.
A) Upregulation of INMDA by LY 379268 (N = 7) was blocked by the GluN2A-antagonist Zn2+ (N = 7) but not by the GluN2B-antagonist Ro 256981 (N = 7) (LY plus Zn2+, 97 1%, n = 6; LY plus Ro 256981, 125 1%, n = 6; LY alone, 135 2%, n = 16). The application of LY 379268 (10nM) is indicated by the shaded region. Zn2+ (300nM) and Ro 256981 (0.5 M) were applied to the bath and to the perfusion solutions containing NMDA/glycine. B) LY 379268 (30nM) treatment increases the tyrosine phosphorylation of immunoprecipitated GluN2A- but not GluN2B-containing NMDARs. The averaged relative density of pTyr for GluN2A (N = 4) and GluN2B (N = 4) obtained under each of the conditions is shown. * Indicates P<0.05, Student's t-test.
Gs-dependent receptors enhance PKA and target Fyn kinase activation to enhance phosphorylation of NMDARs23. Given that Fyn kinase was not activated by LY 379268 and that mGluR2/3 classically signal via inhibition of PKA, we initially confirmed that LY 379268 does indeed inhibit cAMP in treated hippocampal slices. Applications of LY 379268 reduced basal levels of cAMP in a concentration-dependent manner with an IC50 of 10.932nM (Fig. 6a).
Group II metabotropic glutamate receptors inhibit cAMP formation and prevent PKA-mediated phosphorylation of Csk.
A) Dose-response curve showing a dose-dependent inhibition of cAMP formation with increasing concentrations of LY 379268. The IC50 for LY-mediated inhibition of cAMP was: 10.932nM. B) LY 379268 (30nM) treatment decreases the phosphorylation of Csk on its C-terminal Ser364. Below, summary of immunoblot analysis shows the averaged relative density of pCskS396 for control (n = 4) and LY 379268 (n = 4) treatments. * Indicates p<0.05, Student's t-test.
Src is strongly regulated in CA1 hippocampal neurons by C-terminal Src kinase (Csk), which phosphorylates Src on the C-terminal tyrosine Tyr527 and maintains it in an inactive conformation30. Furthermore, the tyrosine kinase activity of Csk is increased by PKA-mediated phosphorylation31, suggesting that a decrease in PKA activity might inhibit Csk and lead to a disinhibition of Src activity. To test this possibility, we first determined whether activation of mGluR2/3 inhibits Csk activity. Treatment of hippocampal membranes with LY379268 led to a significant reduction of Csk activity, as assessed by phosphorylation of Ser364 (Fig. 6b). Furthermore, LY379268 treatment significantly reduced Csk-mediated phosphorylation of Src at its C-terminal regulatory tyrosine Y527 (Fig. 7a). Decreased phosphorylation of Y527 on Src prevents the intramolecular interaction with the SH2 domain and leads to an open conformation of Src32,33. Thus, activation of mGluR2/3 may activate Src through inhibition of its regulatory partner Csk. To determine whether inhibition of PKA may activate Src and occlude the effects of LY379268 on NMDAR currents, we applied a highly selective PKA inhibitor in the patch pipette and examined the modulation of NMDAR currents by LY 379268. Under this condition LY 379268 failed to enhance NMDAR currents (Fig. 7b) suggesting that Src is activated, at least in part, by a reduction in PKA activity.
Inhibition of PKA via group II metabotropic glutamate receptors activates Src kinase.
A) LY 379268 (30nM) treatment to hippocampal slices significantly reduces Csk-mediated phosphorylation of Src at its C-terminal regulatory tyrosine Y527. Decreased phosphorylation of Y527 prevents the intramolecular interaction with the SH2 domain on Src, promoting an open conformation of Src. Below, summary of immunoblot analysis shows the averaged relative density of pSrcY527 for control (n = 4) and LY 379268 (n = 4) treatments. * Indicates p<0.05, Student's t-test. B) The upregulation of NMDAR-currents by LY 379268 (N = 11) was occluded by the PKA inhibitory peptide PKI1422 (N = 9) (LY plus PKI1422, 99 2%, n = 9; LY alone, 135 2%, n = 16). PKI1422 was included inside the patch pipette. The shaded region indicates the period of LY 379268 (10nM) application.
Discussion
We have shown that activation of group II mGluRs enhances postsynaptic NMDAR function in spite of reducing excitatory transmission. Application of the selective mGluR2/3 agonist LY 379268 increases the ratio of NMDA-to-AMPA EPSCs at Schaffer Collateral-CA1 synapses and enhances NMDA-induced currents in acutely isolated CA1 pyramidal neurons. The enhancement of NMDA-evoked currents was mediated by mGluR2/3 as it was inhibited by the selective group II mGluR antagonist LY 341 495. The Src family kinase Src and not Fyn, was required for this potentiation as it was blocked by the selective Src-interfering peptide, Src(4058), but not by the comparable Fyn interfering peptide, Fyn(3957). Furthermore, LY 379268 increased the activity of Src kinase (increased phosphorylation of Tyr-416) but not that of Fyn (phosphorylation of Tyr-420 unchanged), leading to a selective tyrosine phosphorylation of GluN2A- versus GluN2B-containing NMDARs. Unlike Gq-dependent signaling, the potentiation by LY 379268 was insensitive to a blocker of PKC and was occluded by an inhibitor of PKA. This suggests that the mechanism of Src activation by LY 379268 differs substantially from Gq-coupled receptors which signal via sequential activation of PKC, Pyk2 and Src20,22,34.
We provide evidence that group II mGluRs activate Src by inhibition of PKA and Csk activities. Activation of the cAMP-PKA pathway has previously been shown to down regulate Src kinase activity in a Csk-dependent manner31,35. Activation of mGluR2/3 couples to the inhibition of PKA and decreases phosphorylation of Ser364 on Csk, thereby inhibiting Csk activity. This in turn reduces the ability of Csk to phosphorylate the regulatory Y527 on Src and promotes an active conformation of Src. However, we cannot rule out the potential role of activation of an unidentified tyrosine phosphatase, which would also reduce phosphorylation of Src at Tyr527. Src kinase activity can also be directly stimulated by Gi/o36. Activated Gi has been shown to interact directly with the catalytic domain of Src, thus changing the conformation of c-Src and allowing increased accessibility of the active site to substrates. Activation of Src by Gi-protein-coupled receptors has also shown to mediate Ras-dependent activation of mitogen-activated protein kinases (MAPKs) in various cell types37,38. Stimulation of the Gi-coupled -2 adrenergic receptor leads to liberation of G subunits and phospholipase C activation37. The resulting increase in intracellular Ca2+ activates calmodulin and Pyk2, which then activates c-src. Whether or not mGluR2/3 activates such a signaling pathway in parallel with an inhibition of PKA and Csk activities to up regulate Src kinase activity is unknown.
Agonists of group II mGluRs have opposing actions on pre- and post-synaptic sites: decreasing glutamate release presynaptically14 and potentiating NMDAR function postsynaptically (our present findings). Thus, the relative contributions of pre- to post-synaptic effects of mGluR2/3 agonist in vivo could be complicated. One possibility is that reduced glutamate release will prevent the activation of extrasynaptic NMDARs and preferentially activate subsets of synaptic NMDARs. The NMDAR appears to be an important postsynaptic target for mGluR2/3 in the hippocampus. Activation of mGluR2/3 is also reported to decrease the AMPA-component of EPSPs, consistent with a reduction in presynaptic glutamate release39,40. We have shown that mGluR2/3 activation decreases the ratio of AMPA-to-NMDA EPSCs, suggesting that LY 379268 increases the relative contribution of NMDARs to AMPARs in the synapse. The paradoxical effects of mGluR2/3 agonists on presynaptic glutamate release and postsynaptic NMDAR function can also be explained by the activation of extrasynaptic tonic NMDAR conductances. Ambient glutamate release from non-synaptic sources, such as glial cells, induces a tonic NMDA current primarily mediated by extrasynaptic NMDARs41. Given that acutely isolated neurons from the CA1 are heavily enriched in extrasynaptic NMDARs, we propose that LY 379268 may target these extrasynaptic NMDAR currents independently of vesicular glutamate release.
Several lines of evidence suggest that hypofunction of NMDARs underlies the pathophysiology of schizophrenia. Administration of dissociative anesthetics such as phencyclidine and ketamine to healthy volunteers produces behaviours similar to the positive, negative and cognitive symptoms of schizophrenia1,4. Analysis of postmortem hippocampal tissue from schizophrenic patients reveals a decrease in GluN1 mRNA42. Candidate schizophrenia genes such as neuregulin 1 have been shown to promote rapid internalization of NMDARs from the cell surface and reduce whole-cell NMDAR currents43. In postmortem brains of schizophrenia subjects, enhanced ErbB4 signaling by neuregulin 1 mediates a suppression of GluN2A tyrosine phosphorylation, which promotes NMDAR internalization and decreases NMDAR signaling3. Thus, enhancing GluN2A-containing NMDAR function with group II mGluRs may counteract the effects of neuregulin 1 in these individuals and help stabilize NMDARs to the cell surface.
A recent study suggested that dysregulated Src activity mediates NMDAR hypofunction in schizophrenia induced by neuregulin-1-ErbB4 signaling, a candidate schizophrenia pathway44. Activation of neuregulin 1-ErbB4 signaling prevented the Src-induced potentiation of NMDAR-mediated synaptic currents in mouse prefrontal cortex and hippocampus. Thus, normalizing Src-mediated enhancement of NMDARs via activation of group II metabotropic glutamate receptors could represent a therapeutic avenue in the treatment of schizophrenia. The antipsychotic agent clozapine has also been shown to signal through Src kinase to potentiate NMDAR currents in the nucleus accumbens45. Thus, upregulation of Src activity may represent an important mechanism underlying the ability of these agents to relieve both positive and negative symptoms of schizophrenia.
Stimulation of group II mGluRs increases the function of postsynaptic NMDARs in hippocampal CA1 neurons via inhibition of PKA and activation of Src kinase. We propose that inhibition of the cAMP-PKA pathway decreases the activity of Csk, thereby decreasing the phosphorylation of its substrate Src at Tyr527. Reduced phosphorylation of Src at Tyr527 prevents the intramolecular interaction with the SH2 domain and leads to an active conformation of Src. However, we cannot rule out the potential role of activation of an unidentified tyrosine phosphatase, which might also reduce phosphorylation of Src at Tyr527. Up until now, the antipsychotic effects of group II mGluR agonists have been attributed to a decrease in presynaptic glutamate release. Given that schizophrenia is characterized by a hypoglutamatergic state, we propose that enhancing postsynaptic NMDAR function may be more relevant in restoring a balance of glutamatergic signaling in this disease. One candidate schizophrenia pathway leading to hypofunction of NMDARs implicates aberrant Src activity. Thus, we speculate that enhancing Src activity through group II mGluRs may represent one of the mechanisms for the antipsychotic effects of mGluR2/3 agonists.
Methods
Hippocampal slice preparation
Transverse hippocampal slices were prepared from 2- to 3-week old Wistar rats. Following anaesthetization of the animal, the brain was decapitated and quickly removed and placed in ice-cold oxygenated (95% O2, 5% CO2) artificial cerebrospinal fluid (ACSF) containing (in mM): 124 NaCl, 3 KCl, 1.3 MgCl2-6H2O, 2.6 CaCl2, 1.25 NaH2PO4-H2O, 26 NaHCO3, 10 glucose with an osmolarity between 300310 mOsm. Coronal hippocampal slices 300 m thick were prepared using a vibratome (VT100E, Leica). Slices were allowed to recover for at least 1hour in oxygenated ACSF until needed.
Field excitatory postsynaptic potentials (fEPSP) recording from Schaffer collateral-CA1 synapses
fEPSPNMDA were evoked every 30s (0.033Hz) by electrical stimulation (100s duration) delivered to the Schaffer-collateral pathway using a concentric bipolar stimulating electrode (25m exposed tip) and recorded using glass microelectrodes (35M, filled with ACSF) positioned in the stratum radiatum of the CA1 area, when bicuculline methiodide (10M) and CNQX (20M) were present. The input-output relationship and paired-pulse ratio was determined in each slice by varying the stimulus intensity (50200A) and recording the corresponding fEPSP. The stimuli intensity was made as that to evoke 3050% of the maximal fEPSPNMDA. After a 20min stable recording as baseline, LY379268 (30 nM) was bath applied. Signals were amplified (Axoclamp 700B, Molecular Devices, Sunnyvale CA, USA), recorded digitally (Digidata 1440A) and analyzed offline using Clampfit 10.
Whole-cell patch clamp recordings from hippocampal slices
A single slice was transferred to a recording chamber continually superfused with oxygenated ACSF (2ml/min) composed of the following (in mM): 124 NaCl, 3 KCl, 1.25 NaH2PO4, 1.3 MgCl2, 2.6 CaCl2, 26 NaHCO3, 10 glucose and 0.01 bicuculline methiodide (saturated with 95% O25%CO2 at 3133C). Recording electrodes (46M) were filled with internal solution containing (in mM): Cs-gluconate 132.5, CsCl 17.5, HEPES 10, EGTA 0.2, Mg-ATP 2 and GTP 0.3 (pH 7.25, 290 mOsm). Visual patch recordings of CA1 pyramidal neurons were performed using whole-cell configuration with holding potential at 60mV. Synaptic responses were evoked with a concentric bipolar tungsten electrode located about 50m from the cell body layer in CA1 when the neuron was held at 70mV and +40mV, respectively. The AMPAR and NMDAR-mediated EPSC was measured at the peak and 20ms after the start of stimulus artifact at 70mV and +40mV respectively, as previously described46. Signals were amplified using Multiclamp 700B, sampled at 5kHz and analyzed with Clampfit 10 software (Axon Instruments, Foster City, CA).
Cell isolation and whole-cell recordings
CA1 neurons were isolated from the hippocampus of postnatal rats (Wistar, 1422days) using previously described procedures47. To control for variation in response, recordings from control and drug-treated cells were conducted on the same day. The extracellular solution was composed of the following (in mM): 140 NaCl, 1.3 CaCl2, 5 KCl, 25 HEPES, 20 glucose and 0.0005 tetrodotoxin, pH 7.4 (osmolality between 305 and 310 mOsm). Recording electrodes with resistances of 35 M were constructed from borosilicate glass (1.5 M diameter; World Precision Instruments, Sarasota, FL) using a two-stage puller (PP83; Narashige, Tokyo, Japan). Electrodes were filled with intracellular solution composed of the following (in mM): 140 CsF, 11 EGTA, 1 CaCl2, 2 MgCl2, 10 HEPES, 2 tetraethylammonium and 2K2ATP, pH 7.27.3 (osmolality between 290 and 300 mOsm). Where indicated, some drugs were included inside the patch pipette. Recordings were performed at room temperature (2022C). After formation of the whole-cell configuration, the neurons were voltage clamped at 60 mV and lifted into a stream of solution supplied by a computer-controlled, multi-barreled perfusion system (SF-77 B, Warner Instrument Corporation). The exchange time for solutions was ~3050ms. To monitor access resistance, a voltage step of 10mV was made before each application of NMDA. If series resistance was increased to >20M, the cell was discarded. Currents were recorded using AxoPatch 1D amplifier. Data were filtered at 2kHz and digitized at 10kHz using Clampex software.
Zn2+-buffered solutions
The tricine-buffered zinc solutions were prepared according to the empirically established binding constant by adding into 10mM tricine and as previously described48. The amount of ZnCl2 required can be calculated based on the following formula: Znfree = ZnCl2total/300. (At pH 7.3; with 10mM tricine for Znfree = 300nM).
Immunoprecipitation and Western blotting
Hippocampal slices were prepared from Wistar rats (PN 1520) and incubated with ACSF saturated with 95% O2 and 5% CO2 for at least 1hour at room temperature. This was followed by treatment with LY 379268 (30nM for 20min) or vehicle control. For experiments testing Src(4058), Fyn(3957) or mGluR2/3 inhibition, the slices were pretreated with TAT-Src4058 (10 M), or TAT-Fyn3957 (10 M), or LY 341495 (30nM) 30minutes before drug exposures. After three washes with cold PBS, slices were homogenized in ice-cold RIPA buffer (50mM Tris-HCl, pH 7.4, 150mM NaCl, 1mM EDTA, 0.1% SDS, 0.5% Triton X-100 and 1% Sodium Deoxycholate) supplemented with 1mM sodium orthovanadate and 1% protease inhibitor cocktail, 1% protein phosphatases inhibitor cocktails and subsequently spun at 16 000r.c.f. for 30minutes at 4C (Eppendorf Centrifuge 5414R). The supernatant was collected and kept at 70C. For immunoprecipitation, the sample containing 500 g protein was incubated with antibodies (see below) at 4C and gently shaken overnight. Antibodies used for immunoprecipitation were anti-GluN2A and anti-GluN2B (3 g, rabbit IgG; Enzo Life Sciences, PA), anti-Src (1:500, mouse IgG, Cell Signaling Technology; Danvers, MA) and anti-Fyn (1:200, mouse IgG; Santa Cruz Biotechnology, Santa Cruz, CA). The immune complexes were collected with 20 l of protein A/G Sepharose beads for 2h at 4C. Immunoprecipitates were then washed three times with ice-cold PBS, resuspended in 2-x Laemmli sample buffer and boiled for 5minutes. These samples were subjected to SDS-PAGE and transferred to a nitrocellulose membrane. The blotting analysis was performed by repeated stripping and successive probing with antibodies: anti-pY(4G10) (1:2000, mouse IgG; Millipore Corp., Billerica, MA), GluN2A or GluN2B (1:1000, rabbit IgG; Cell Signaling Technology, Danvers, MA), pSrcY416 (1:1500, rabbit IgG; Cell Signaling Technology, Danvers, MA), pSrcY527 (1:1000, rabbit IgG; Cell Signaling Technology, Danvers, MA), pCskS364 (1:500, rabbit IgG; Antibodies-Online, Atlanta, GA) and pGSKSer9 (1:1000; rabbit IgG; Cell Signaling Technology, Danvers, MA).
cAMP assay
Hippocampal slices were prepared from Wistar rats (23week old) and incubated in ACSF saturated with 95% O2 and 5% CO2 for at least 1h at room temperature. Hippocampal tissue was then treated with 30nM LY 379268 or vehicle control for 20minutes. The treated tissue was then washed with cold ACSF 3times and frozen immediately in liquid nitrogen. Equal amounts of frozen tissue were homogenized in 10 volumes of 0.1M HCl and the debris was centrifuged at 10, 000rpm for 10min at 4C (Eppendorf Centrifuge 5415R). Cyclic AMP levels were determined using the cAMP Immunoassay kit (Assay Designs, Ann Arbor, Michigan, USA). In order to increase the sensitivity of cAMP measurement, the supernatant was diluted 10-fold with distilled water. For the assay, 100 l of the diluted sample, 50 l of blue conjugate and 50 l of yellow antibody were each added to the bottom of appropriate wells sequentially and the reaction was incubated for 2hours on a plate shaker at room temperature. The standards were prepared following the protocol supplied by the company. After incubation, the wells were emptied and washed 3times with 400 l of wash buffer. 200 l of substrate solution was then added into each well. The reaction was stopped by adding 50 l of stop solution and incubated at room temperature for 1hour without shaking. The optical density of each well was then determined with a microplate reader set to 405nm.
Drugs and peptides
The sources of drugs for this study are as follows: LY 379268, LY 341495, Ro 25-6981, MPEP hydrochloride, PKI1422 amide (Tocris, Minneapolis, MN), TDZD-8, tricine, ZnCl2, NMDA, glycine (Sigma, St. Louis, MO). Src(4058) was provided by Dr. MW Salter (Hospital for Sick Children, Toronto, Ontario). The Fyn(3957) peptide was synthesized by the Advanced Protein Technology Centre (Toronto, Ontario, Canada) with the following sequence: YPSFGVTSIPNYNNFHAAG, Fyn amino acids 3957. We attached both Src(4058) and Fyn(3957) to Tat transduction domains (YGRLLRQRRR), which allowed us to apply these membrane permeant forms of Src versus Fyn interfering peptides to hippocampal slices for biochemical experiments.
Animal care
All animal experimentation was conducted in accordance with the Policies on the Use of Animals at the University of Western Ontario and approved by the Animal Care Committee of the University of Western Ontario.
Statistics
All population data are expressed as mean SD. Student's t-test was used to compare between two groups and one-way ANOVA with Tukey's post-hoc comparison was used to compare multiple groups.
References
Tsai, G. & Coyle, J. T. Glutamatergic mechanisms in schizophrenia. Annu Rev Pharmacol Toxicol 42, 165 179 (2002).
Pilowsky, L. S. et al. First in vivo evidence of an NMDA receptor deficit in medication-free schizophrenic patients. Mol Psychiatry 11, 118 119 (2006).
Hahn, C. G. et al. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med 12, 824 828 (2006).
Krystal, J. H. et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive and neuroendocrine responses. Arch Gen Psychiatry 51, 199 214 (1994).
Javitt, D. C. & Zukin, S. R. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 148, 1301 1308 (1991).
Harrison, P. J. & Weinberger, D. R. Schizophrenia genes, gene expression and neuropathology: on the matter of their convergence. Mol Psychiatry 10, 4068; image 45 (2005).
Imre, G. The preclinical properties of a novel group II metabotropic glutamate receptor agonist LY379268. CNS Drug Rev 13, 444 464 (2007).
Patil, S. T. et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med 13, 1102 1107 (2007).
Moghaddam, B. & Adams, B. W. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science 281, 1349 1352 (1998).
Galici, R., Echemendia, N. G., Rodriguez, A. L. & Conn, P. J. A selective allosteric potentiator of metabotropic glutamate (mGlu) 2 receptors has effects similar to an orthosteric mGlu2/3 receptor agonist in mouse models predictive of antipsychotic activity. J Pharmacol Exp Ther 315, 1181 1187 (2005).
Olszewski, R. T. et al. Phencyclidine and dizocilpine induced behaviors reduced by N-acetylaspartylglutamate peptidase inhibition via metabotropic glutamate receptors. Biol Psychiatry 63, 86 91 (2008).
Crook, J. M., Akil, M., Law, B. C., Hyde, T. M. & Kleinman, J. E. Comparative analysis of group II metabotropic glutamate receptor immunoreactivity in Brodmann's area 46 of the dorsolateral prefrontal cortex from patients with schizophrenia and normal subjects. Mol Psychiatry 7, 157 164 (2002).
Wright, R. A. et al. CNS distribution of metabotropic glutamate 2 and 3 receptors: Transgenic mice and (3)HLY459477 autoradiography. Neuropharmacology (2012).
Niswender, C. M. & Conn, P. J. Metabotropic glutamate receptors: physiology, pharmacology and disease. Annu Rev Pharmacol Toxicol 50, 295 322 (2010).
Anwyl, R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev 29, 83 120 (1999).
Manahan-Vaughan, D. Group 1 and 2 metabotropic glutamate receptors play differential roles in hippocampal long-term depression and long-term potentiation in freely moving rats. J Neurosci 17, 3303 3311 (1997).
Weinberger, D. R., Berman, K. F., Suddath, R. & Torrey, E. F. Evidence of dysfunction of a prefrontal-limbic network in schizophrenia: a magnetic resonance imaging and regional cerebral blood flow study of discordant monozygotic twins. Am J Psychiatry 149, 890 897 (1992).
Marsh, L., Suddath, R. L., Higgins, N. & Weinberger, D. R. Medial temporal lobe structures in schizophrenia: relationship of size to duration of illness. Schizophr Res 11, 225 238 (1994).
Lu, W. Y. et al. G-protein-coupled receptors act via protein kinase C and Src to regulate NMDA receptors. Nat Neurosci 2, 331 338 (1999).
Kotecha, S. A. et al. Co-stimulation of mGluR5 and N-methyl-D-aspartate receptors is required for potentiation of excitatory synaptic transmission in hippocampal neurons. J Biol Chem 278, 27742 27749 (2003).
MacDonald, J. F., Kotecha, S. A., Lu, W. Y. & Jackson, M. F. Convergence of PKC-dependent kinase signal cascades on NMDA receptors. Curr Drug Targets 2, 299 312 (2001).
Macdonald, D. S. et al. Modulation of NMDA receptors by pituitary adenylate cyclase activating peptide in CA1 neurons requires G alpha q, protein kinase C and activation of Src. J Neurosci 25, 11374 11384 (2005).
Yang, K. et al. Metaplasticity gated through differential regulation of GluN2A versus GluN2B receptors by Src family kinases. EMBO J (2011).
Monn, J. A. et al. Synthesis, pharmacological characterization and molecular modeling of heterobicyclic amino acids related to (+)2-aminobicyclo3.1.0 hexane-2,6-dicarboxylic acid (LY354740): identification of two new potent, selective and systemically active agonists for group II metabotropic glutamate receptors. J Med Chem 42, 1027 1040 (1999).
Skeberdis, V. A. et al. Protein kinase A regulates calcium permeability of NMDA receptors. Nat Neurosci 9, 501 510 (2006).
Gingrich, J. R. et al. Unique domain anchoring of Src to synaptic NMDA receptors via the mitochondrial protein NADH dehydrogenase subunit 2. Proc Natl Acad Sci U S A 101, 6237 6242 (2004).
Smart, J. E. et al. Characterization of sites for tyrosine phosphorylation in the transforming protein of Rous sarcoma virus (pp60v-src) and its normal cellular homologue (pp60c-src). Proc Natl Acad Sci U S A 78, 6013 6017 (1981).
Cheng, S. H. et al. Structural elements that regulate pp59c-fyn catalytic activity, transforming potential and ability to associate with polyomavirus middle-T antigen. J Virol 65, 170 179 (1991).
Paoletti, P., Ascher, P. & Neyton, J. High-affinity zinc inhibition of NMDA NR1-NR2A receptors. J Neurosci 17, 5711 5725 (1997).
Nada, S., Okada, M., MacAuley, A., Cooper, J. A. & Nakagawa, H. Cloning of a complementary DNA for a protein-tyrosine kinase that specifically phosphorylates a negative regulatory site of p60c-src. Nature 351, 69 72 (1991).
Vang, T. et al. Activation of the COOH-terminal Src kinase (Csk) by cAMP-dependent protein kinase inhibits signaling through the T cell receptor. J Exp Med 193, 497 507 (2001).
Liu, X. et al. Regulation of c-Src tyrosine kinase activity by the Src SH2 domain. Oncogene 8, 1119 1126 (1993).
Roussel, R. R., Brodeur, S. R., Shalloway, D. & Laudano, A. P. Selective binding of activated pp60c-src by an immobilized synthetic phosphopeptide modeled on the carboxyl terminus of pp60c-src. Proc Natl Acad Sci U S A 88, 10696 10700 (1991).
Heidinger, V. et al. Metabotropic glutamate receptor 1-induced upregulation of NMDA receptor current: mediation through the Pyk2/Src-family kinase pathway in cortical neurons. J Neurosci 22, 5452 5461 (2002).
Abrahamsen, H., Vang, T. & Taskn, K. Protein kinase A intersects SRC signaling in membrane microdomains. J Biol Chem 278, 17170 17177 (2003).
Ma, Y. C., Huang, J., Ali, S., Lowry, W. & Huang, X. Y. Src tyrosine kinase is a novel direct effector of G proteins. Cell 102, 635 646 (2000).
Della Rocca, G. J. et al. Ras-dependent mitogen-activated protein kinase activation by G protein-coupled receptors. Convergence of Gi- and Gq-mediated pathways on calcium/calmodulin, Pyk2 and Src kinase. J Biol Chem 272, 19125 19132 (1997).
Luttrell, L. M. et al. Role of c-Src tyrosine kinase in G protein-coupled receptor- and Gbetagamma subunit-mediated activation of mitogen-activated protein kinases. J Biol Chem 271, 19443 19450 (1996).
Farazifard, R. & Wu, S. H. Metabotropic glutamate receptors modulate glutamatergic and GABAergic synaptic transmission in the central nucleus of the inferior colliculus. Brain Res 1325, 28 40 (2010).
Poisik, O. et al. Metabotropic glutamate receptor 2 modulates excitatory synaptic transmission in the rat globus pallidus. Neuropharmacology 49 Suppl 1, 57 69 (2005).
Le Meur, K., Galante, M., Angulo, M. C. & Audinat, E. Tonic activation of NMDA receptors by ambient glutamate of non-synaptic origin in the rat hippocampus. J Physiol 580, 373 383 (2007).
Gao, X. M. et al. Ionotropic glutamate receptors and expression of N-methyl-D-aspartate receptor subunits in subregions of human hippocampus: effects of schizophrenia. Am J Psychiatry 157, 1141 1149 (2000).
Gu, Z., Jiang, Q., Fu, A. K., Ip, N. Y. & Yan, Z. Regulation of NMDA receptors by neuregulin signaling in prefrontal cortex. J Neurosci 25, 4974 4984 (2005).
Pitcher, G. M. et al. Schizophrenia susceptibility pathway neuregulin 1-ErbB4 suppresses Src upregulation of NMDA receptors. Nat Med 17, 470 478 (2011).
Wittmann, M., Marino, M. J., Henze, D. A., Seabrook, G. R. & Conn, P. J. Clozapine potentiation of N-methyl-D-aspartate receptor currents in the nucleus accumbens: role of NR2B and protein kinase A/Src kinases. J Pharmacol Exp Ther 313, 594 603 (2005).
von Engelhardt, J. et al. CKAMP44: a brain-specific protein attenuating short-term synaptic plasticity in the dentate gyrus. Science 327, 1518 1522 (2010).
Wang, L. Y. & MacDonald, J. F. Modulation by magnesium of the affinity of NMDA receptors for glycine in murine hippocampal neurones. J Physiol 486 (Pt 1), 83 95 (1995).
Nozaki, C. et al. Zinc alleviates pain through high-affinity binding to the NMDA receptor NR2A subunit. Nat Neurosci 14, 1017 1022 (2011).
Acknowledgements
This study was supported by a grant to JFM by CIHR. We thank Dr. MW Salter for the Src interfering peptide Src(4058).
Author information
Authors and Affiliations
Contributions
CT designed and performed whole-cell voltage clamp recordings from acutely isolated hippocampal neurons. YFX designed and performed whole-cell voltage clamp recordings from CA1 pyramidal neurons in slice. GL designed and performed all biochemical experiments. JFM conceptualized and supervised the project and contributed to the design of the experiments. CHT and JFM wrote the manuscript. All authors read and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Trepanier, C., Lei, G., Xie, YF. et al. Group II metabotropic glutamate receptors modify N-methyl-D-aspartate receptors via Src kinase. Sci Rep 3, 926 (2013). https://doi.org/10.1038/srep00926
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep00926
This article is cited by
-
Metabotropic glutamate receptor subtype 3 gates acute stress-induced dysregulation of amygdalo-cortical function
Molecular Psychiatry (2019)
-
Physiological activation of mGlu5 receptors supports the ion channel function of NMDA receptors in hippocampal LTD induction in vivo
Scientific Reports (2018)
-
Targeting metabotropic glutamate receptors for novel treatments of schizophrenia
Molecular Brain (2017)
-
Dopamine promotes NMDA receptor hypofunction in the retina through D1 receptor-mediated Csk activation, Src inhibition and decrease of GluN2B phosphorylation
Scientific Reports (2017)
-
Contribution of an SFK-Mediated Signaling Pathway in the Dorsal Hippocampus to Cocaine-Memory Reconsolidation in Rats
Neuropsychopharmacology (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.